- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


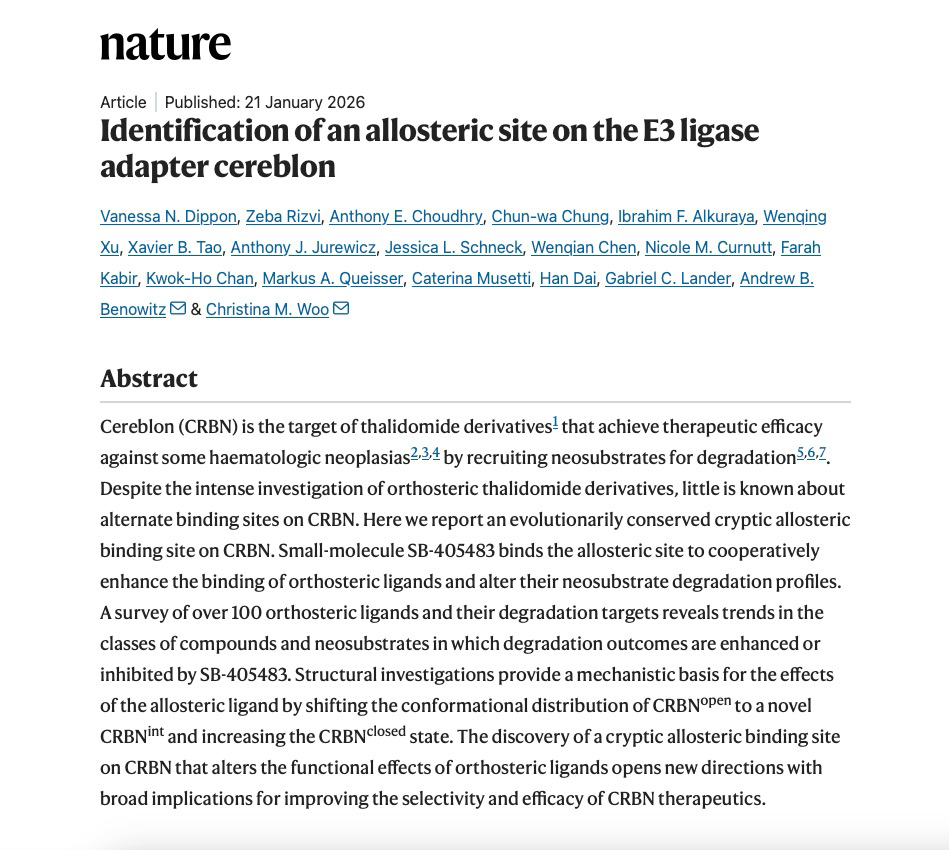
Cereblon (CRBN) is the target of thalidomide derivatives that achieve therapeutic efficacy against some haematologic neoplasias by recruiting neosubstrates for degradation. Despite the intense investigation of orthosteric thalidomide derivatives, little is known about alternate binding sites on CRBN.
Recent research published in Nature has advanced the field by identifying and characterizing an evolutionarily conserved allosteric binding site on the E3 ubiquitin ligase adaptor protein CRBN. This study provides a novel theoretical and structural framework to refine the selectivity and functional profiles of CRBN-targeted therapeutics. The study, entitled “Identification of an allosteric site on the E3 ligase adapter cereblon”, represents a joint effort by several involving multiple research institutions, including Harvard University, Scripps Research, and GlaxoSmithKline (GSK). Viva Biotech very horned to participated in this collaboration as a partner, contributing to protein preparation and crystallographic structure determination, and providing robust structural biology support for this discovery.
Dr. Han Dai, Chief Innovation Officer of Viva Biotech and Head of Viva BioInnovator, participated in this research during his tenure at GSK and collaborated with the structural biology team led by Dr. Dongming Qian, Vice President of Protein and Structural Biology at Viva Biotech. They completed the protein preparation and crystallographic structure determination, providing critical support for this key scientific discovery.
(Source: Nature website)
Cereblon is an E3 ubiquitin ligase substrate adapter protein identified as the primary binding target of thalidomide. Thalidomide and its derivatives engage CRBN at the thalidomide-binding domain (TBD) and promote the recruitment of chemically induced substrates (‘neosubstrates’) for ubiquitination and proteasomal degradation. Despite these intensive efforts focused on characterizing the TBD and its endogenous substrate recognition mechanisms, our understanding of other binding pockets on CRBN that may regulate substrate and neosubstrate degradation outcomes is limited. In this research, an evolutionarily conserved cryptic allosteric binding site on CRBN. Small-molecule SB-405483 binds the allosteric site to cooperatively enhance the binding of orthosteric ligands and alter their neosubstrate degradation profiles.
During this research, the Viva Biotech structural biology team first constructed CRBN/DDB1 clones. By Leveraging a well-established insect cell expression system and iteratively optimizing purification protocols, the team successfully produced CRBN/DDB1 complex proteins with high purity and homogeneity. Utilizing a proprietary high-throughput crystallization platform, the team screened diverse protein–compound combinations across tens of thousands of conditions, ultimately obtaining co-crystals of the CRBN/DDB1 complex with the small molecule SB-405483. Subsequently, X-ray diffraction analysis enabled the determination of a high-resolution crystal structure at approximately 2.4 Å. These high-quality structural data elucidated, for the first time, the molecular mechanism by which SB-405483 binds to the allosteric site on CRBN, providing a critical structural foundation for subsequent drug discovery and development efforts.
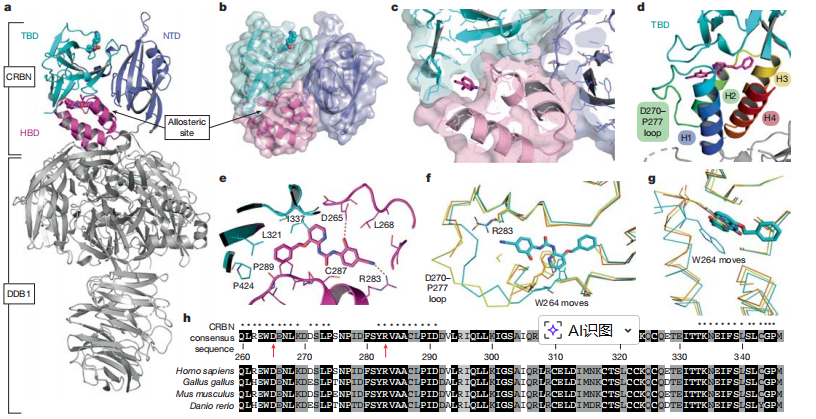
Figure 1: Crystal structure of human CRBN–DDB1 with allosteric SB-405483 compound (magenta) and lenalidomide (cyan).
For further details on the paper, please refer to:
Dippon, V.N., Rizvi, Z., Choudhry, A.E., Chung, C., Alkuraya, I.F., Xu, W., Tao, X.B., Jurewicz, A.J., Schneck, J.L., Chen, W., Curnutt, N.M., Kabir, F., Chan, K.-H., Queisser, M.A., Musetti, C., Dai, H., Lander, G.C., Benowitz, A.B. and Woo, C.M. (2026). Identification of an allosteric site on the E3 ligase adapter cereblon. Nature. doi: https://doi.org/10.1038/s41586-025-09994-w
Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us