Viva Biotech has a professional team specializing in preclinical druggability and functionality testing of antibody drugs, offering comprehensive services in immunocyte differentiation and inducted amplification, in vitro efficacy testing of antibody drugs, and development and validation of other antibody drug immunofunctional experimental methods.

-
Immunocyte Differentiation and Inducted Amplification PlatformT cell activation experimentsTreg cell amplification and functional verification servicesDendritic cell (iDC/mDC) induction servicesMacrophage induction servicesNK cell activation and amplification services
-
In Vitro Efficacy Testing Platform for Antibody DrugsAntibody-dependent cellular cytotoxicity (ADCC) experimentsAntibody-dependent cellular phagocytosis (ADCP)Complement-dependent cytotoxicity (CDC) experimentsMixed lymphocyte reaction (MLR)T cell/NK cell killing assayAntibody affinity and blockade activity assayAntibody internalization activity assayADC drug-mediated cytotoxicity assayMulticellular inflammatory cytokine release assayVaccine immunogenicity assessment
-
Other Functional Verification and Customized Experiments for BiologicsExtracellular Cytokine Secretion AssayCell Cycle AnalysisApoptosis AssayHTRF AssayAlphaLISA Assay
-
Immunocyte Differentiation and Inducted Amplification Platform
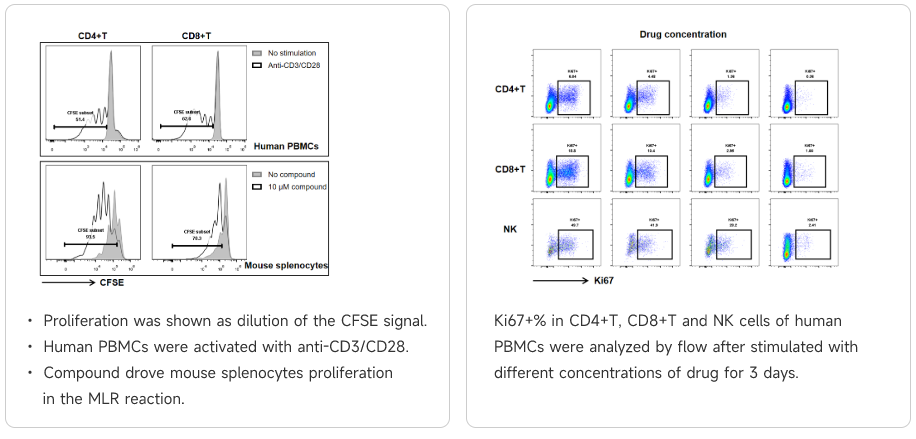
T cell activation experiments
T cells primarily mediate cellular immunity and regulate the body's immune response. The T cell immune response is the most critical host response in controlling tumor growth and development.
Data Presentation
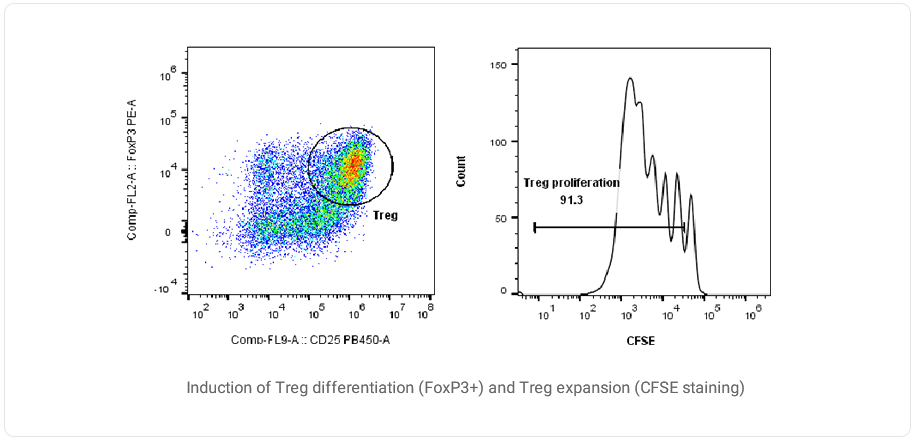
Treg Cell Amplification and Functional Verification
Regulatory T cells (Treg) are one of the important factors in maintaining the body's immune tolerance. They can also actively regulate the immune response to foreign antigens and antigen-related self-changes.
Data Presentation
Dendritic Cell (iDC/mDC) Induction
DC cells are a group of professional antigen-presenting cells with super-strong ability to activate primary T cells. They are the initiators of adaptive immune responses. DC cells have great plasticity, which can play an immune activating role and induce immune tolerance, and have multiple functions such as antigen presentation, immune tolerance, T cell polarization, B cell regulation, and participation in innate immune response.
Data Presentation
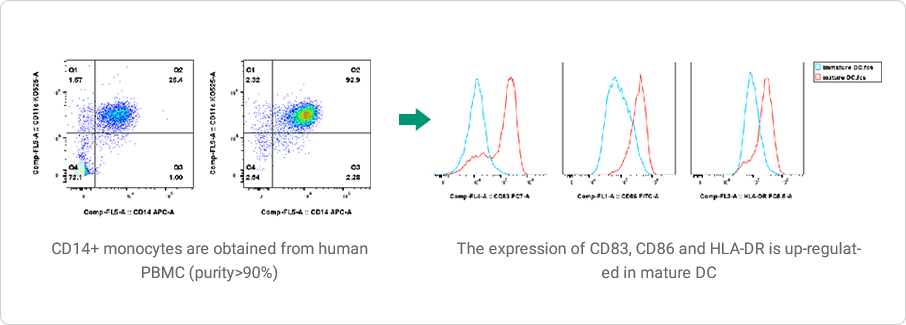
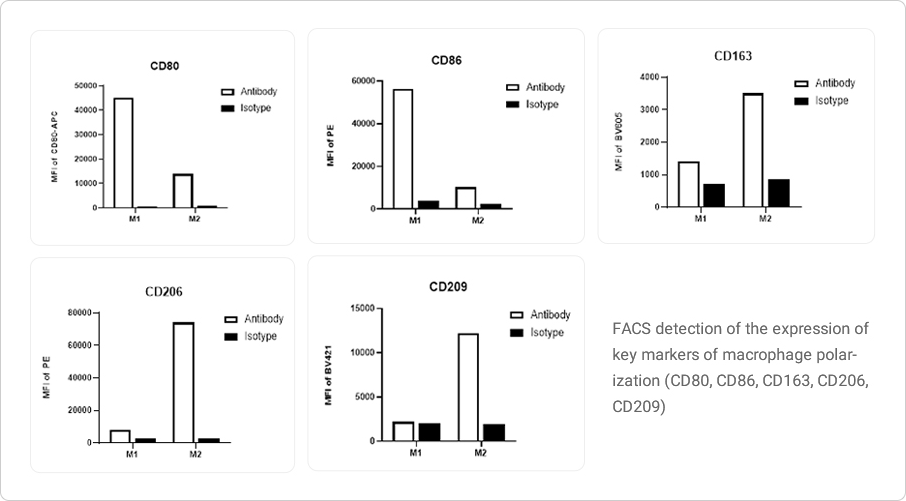
Macrophage induction
M0 macrophages are derived from monocytes differentiated by M-CSF (macrophage colony-stimulating factor). Depending on the activation mode of M0 macrophages, they can be further divided into two categories: classical activated M1 macrophages and alternatively activated M2 macrophages. M2 macrophages mainly perform immune regulatory functions and promote Th2-mediated immune responses.
Data Presentation
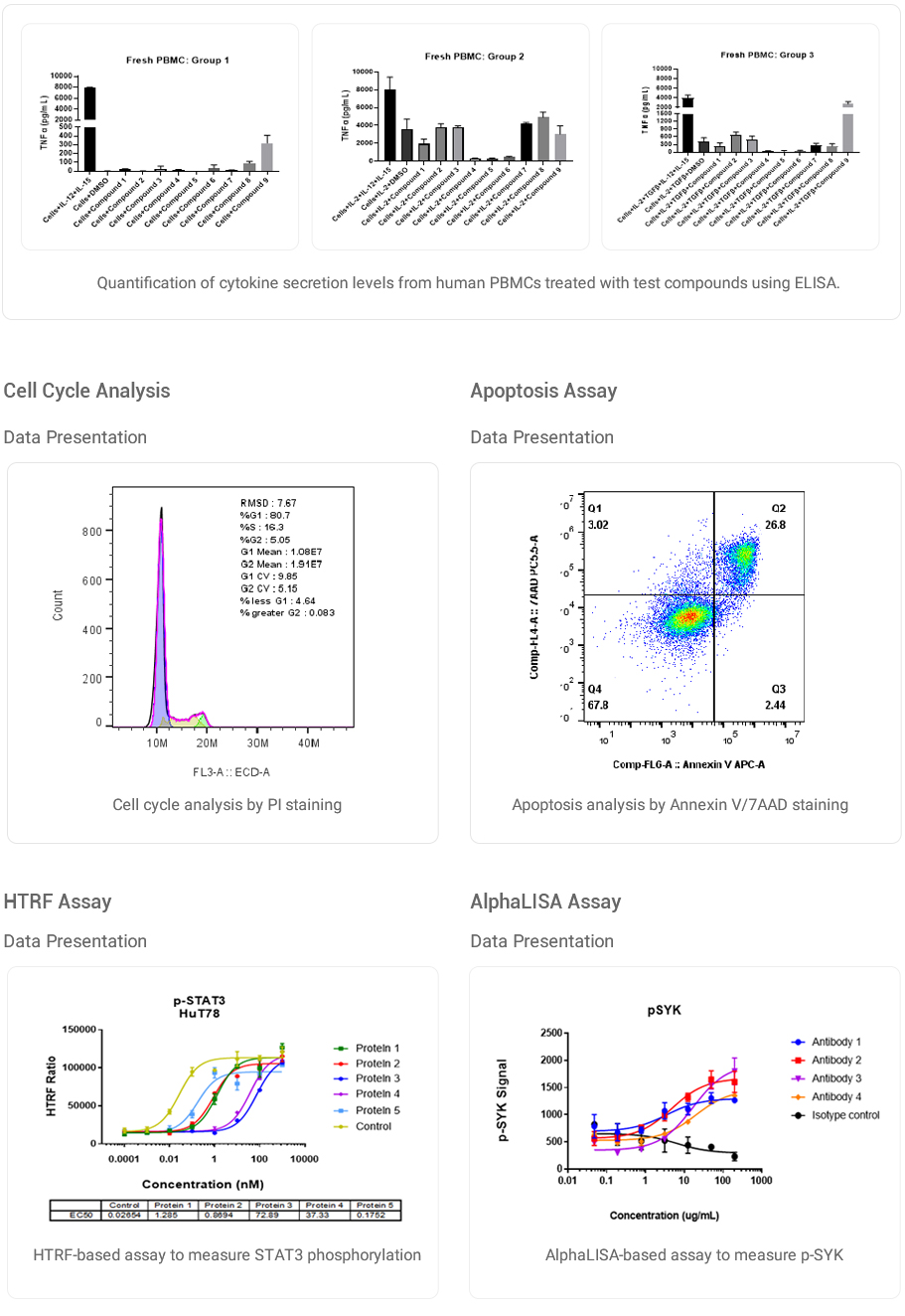
NK Cell Activation and Amplification
Natural killer (NK) cells are important immune cells in the body. They are not only related to anti-tumor, anti-viral infection, and immune regulation, but also involved in the occurrence of hypersensitivity reactions and autoimmune diseases in certain cases, and can recognize target cells and cytotoxic agents.
Data Presentation
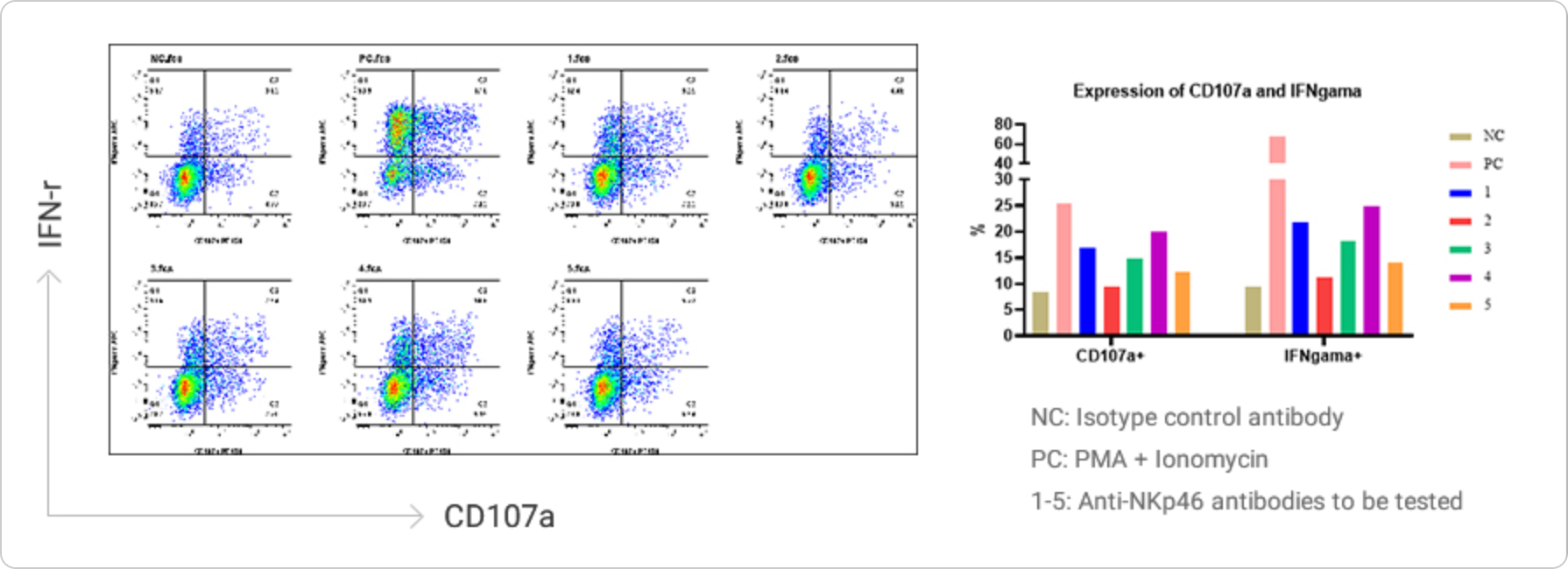
NK cells were treated with plate-bound anti-NKp46 antibody, GolgiPlug, and anti-CD107a fluorescent antibody at 37°C for 4-6 hours. Then the cells were collected, fixed and permeabilized, and intracellular IFN-γ was stained and analyzed by flow cytometry. NK cell degranulation was assessed by surface expression of CD107a.
-
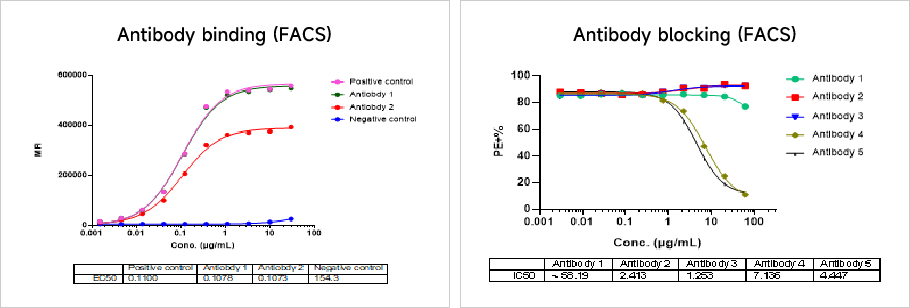
Antibody Drug In Vitro Efficacy Testing PlatformAntibody affinity and blockade activity assay
Data Presentation
Antibody Internalization Activity AssayThe internalization efficiency of antibody therapeutics can be assessed by multiple approaches, including changes in cell surface antibody binding levels, fluorescently-labeled antibodies, and DT3C-conjugated antibodies.
Data Presentation
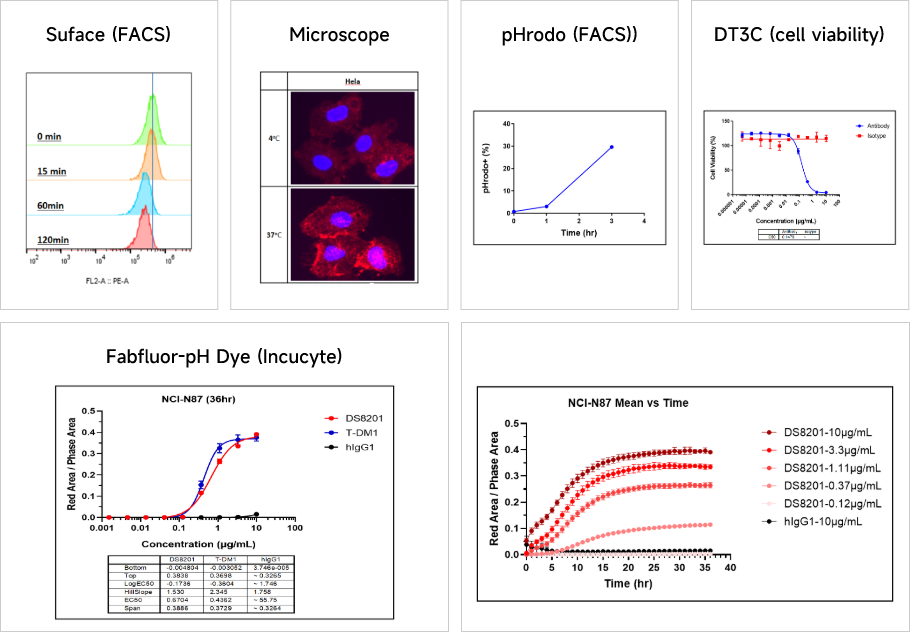
Cytotoxicity AssaysThe internalization efficiency of antibody therapeutics can be assessed by multiple approaches, including changes in cell surface antibody binding levels, fluorescently-labeled antibodies, and DT3C-conjugated antibodies.
Data Presentation
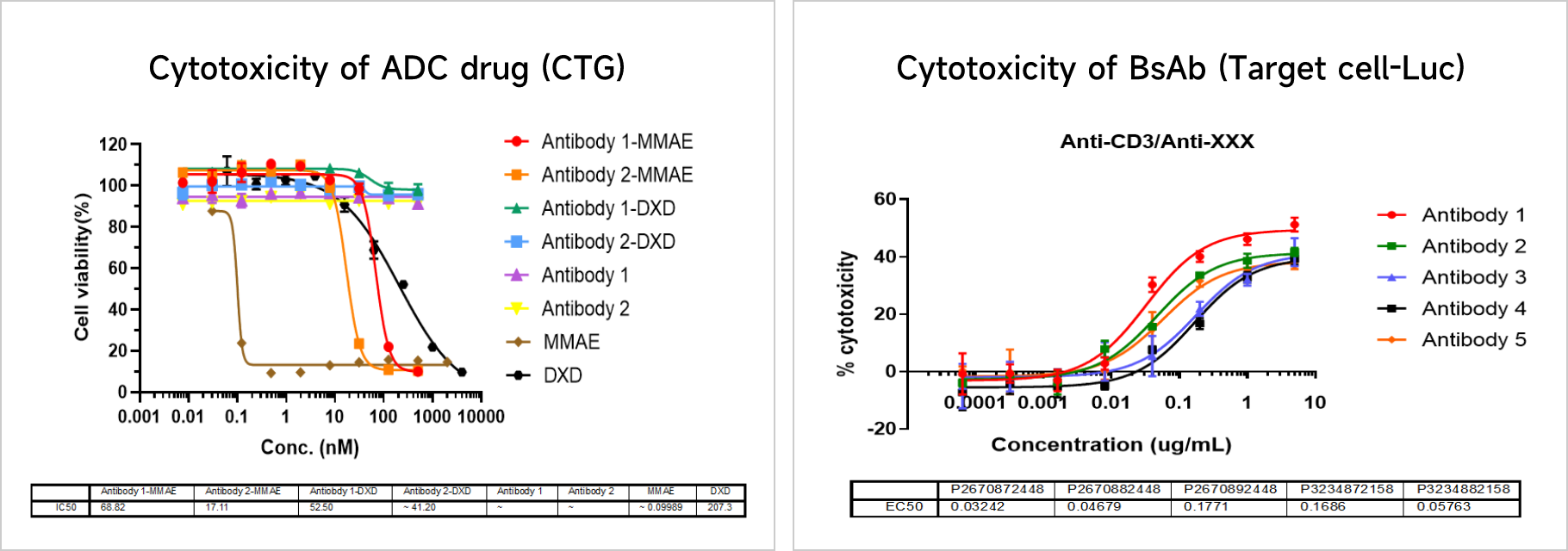
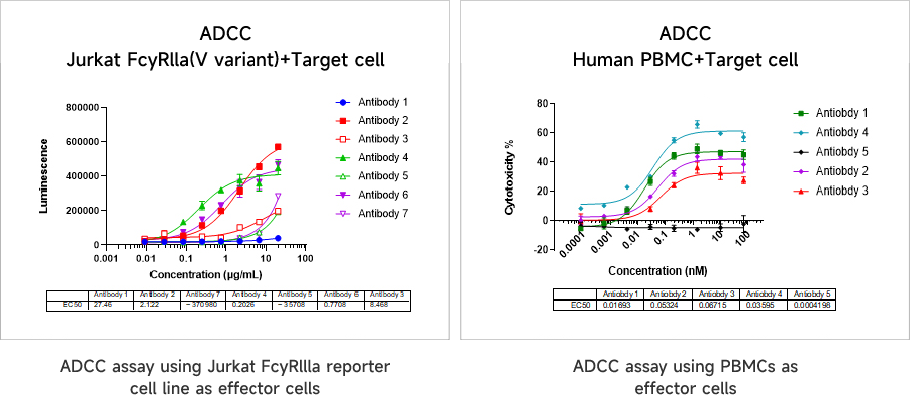
Antibody-Dependent Cellular Cytotoxicity (ADCC) AssayThe internalization efficiency of antibody therapeutics can be assessed by multiple approaches, including changes in cell surface antibody binding levels, fluorescently-labeled antibodies, and DT3C-conjugated antibodies.
Data Presentation
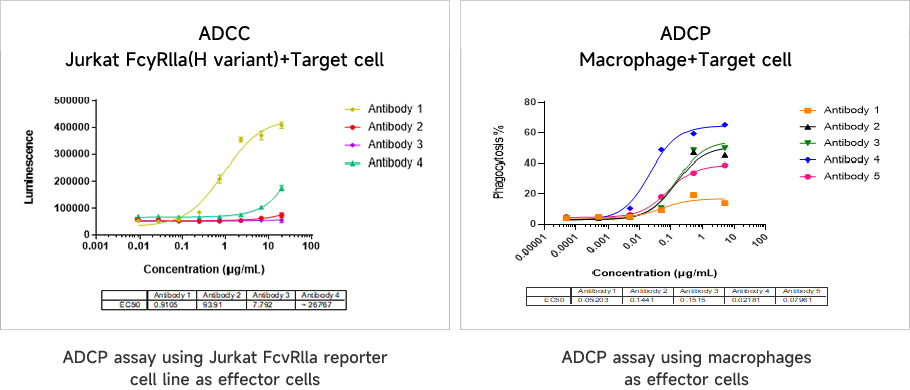
 Antibody-Dependent Cellular Phagocytosis (ADCP) Assay
Antibody-Dependent Cellular Phagocytosis (ADCP) AssayADCP assays are an important part of antibody drug efficacy assessment and are a well-established and robust method for evaluating macrophage-mediated induction and phagocytosis.
Data Presentation
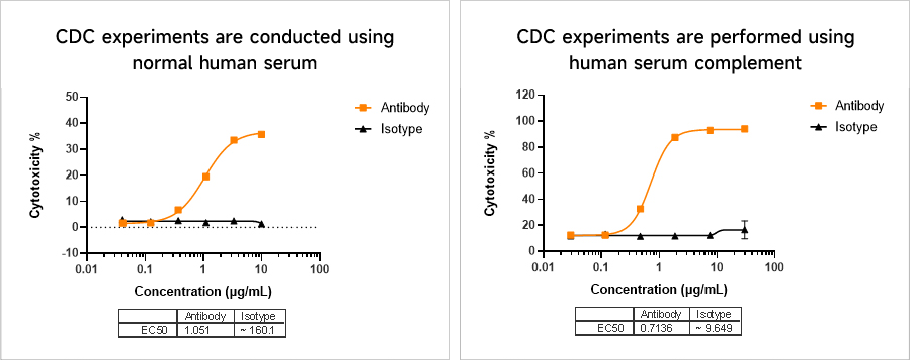
 Complement-Dependent Cytotoxicity (CDC) Assay
Complement-Dependent Cytotoxicity (CDC) AssayComplement-dependent cytotoxicity involves the binding of the Fc region of an antibody to complement molecules and activating them. The Fab region of the antibody binds to the target cell, subsequently forming a membrane attack complex, ultimately leading to the lysis of the target cell.
Data Presentation
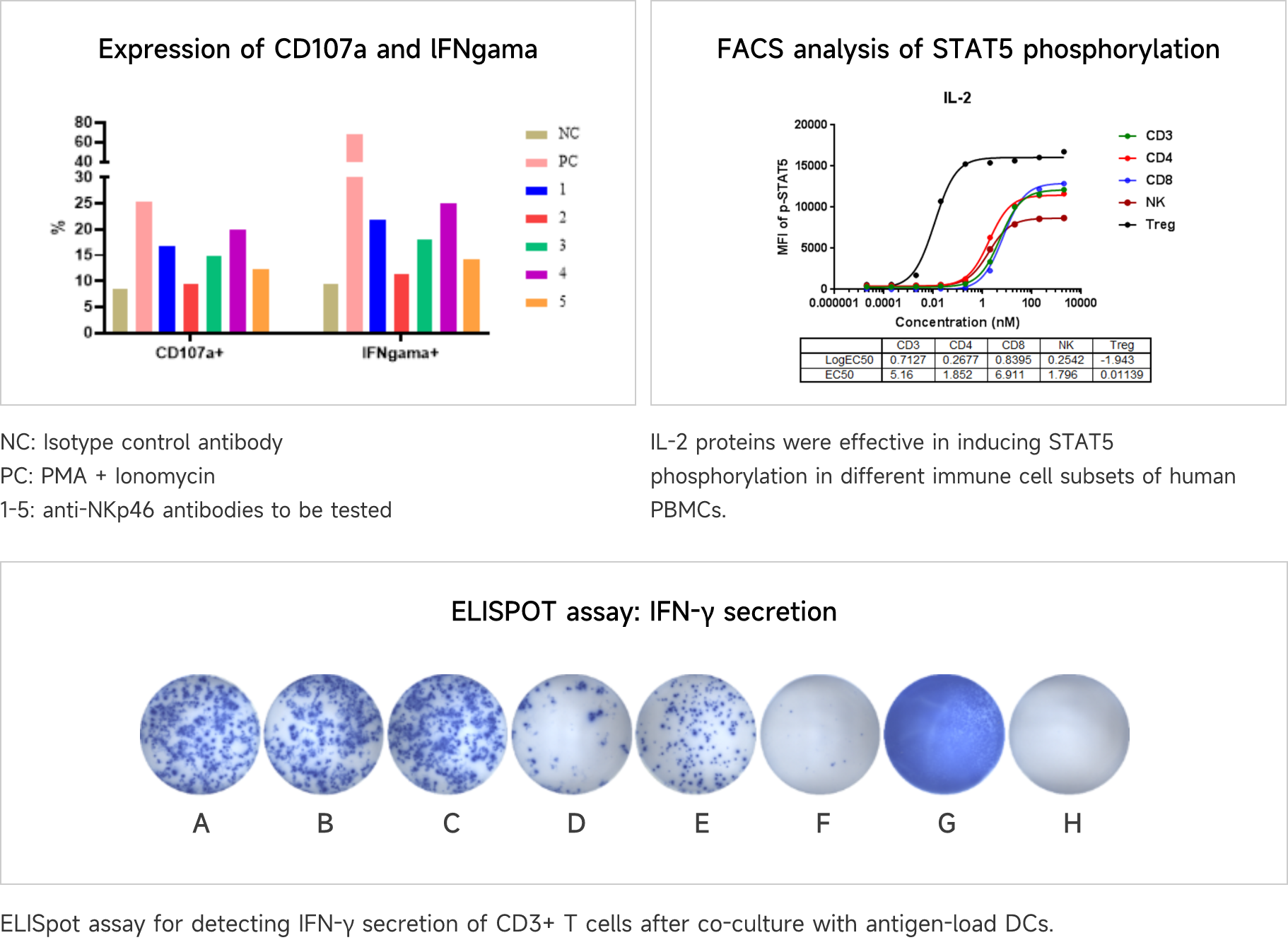
 Mixed Lymphocyte Reaction (MLR)
Mixed Lymphocyte Reaction (MLR)To mimic the ability of DCs to activate or suppress T cell proliferation and cytokine secretion in vitro is a very important approach to understanding the functionality of compounds that act as either agonists or antagonists.
Data Presentation
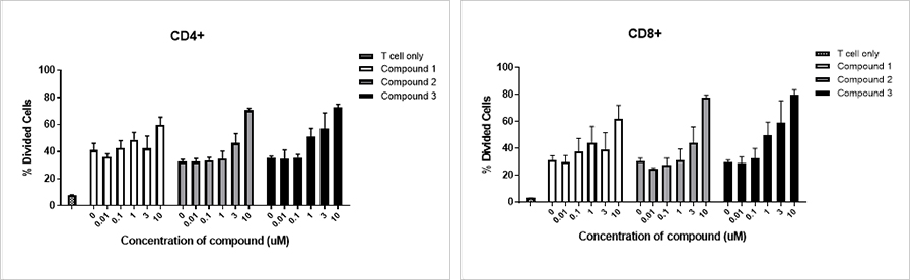
Compounds were tested for their ability to drive T cell activation and proliferation in an MLR. T cell activation was analyzed by flow cytometry for CD4, CD8, and CellTrace Violet
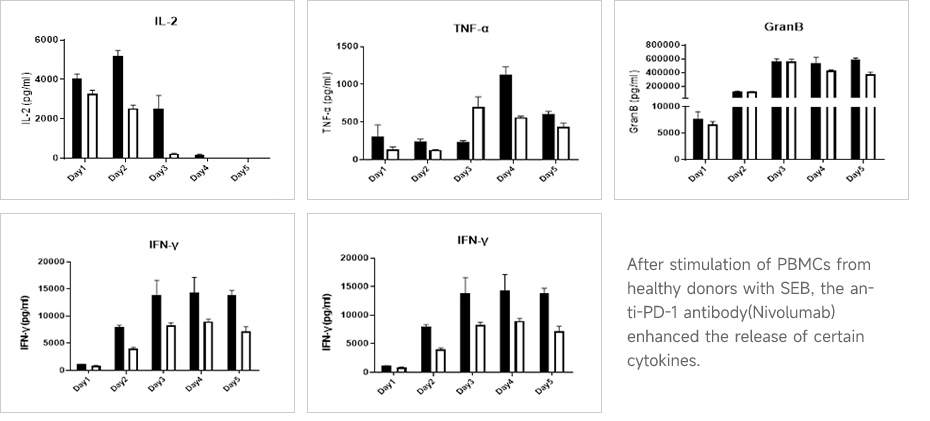
Multicellular Inflammatory Cytokine Release AssayBased on Cytometric Bead Array (CBA), multiple cellular inflammatory cytokine releases are detected to evaluate the safety of antibody drugs.
Data Presentation
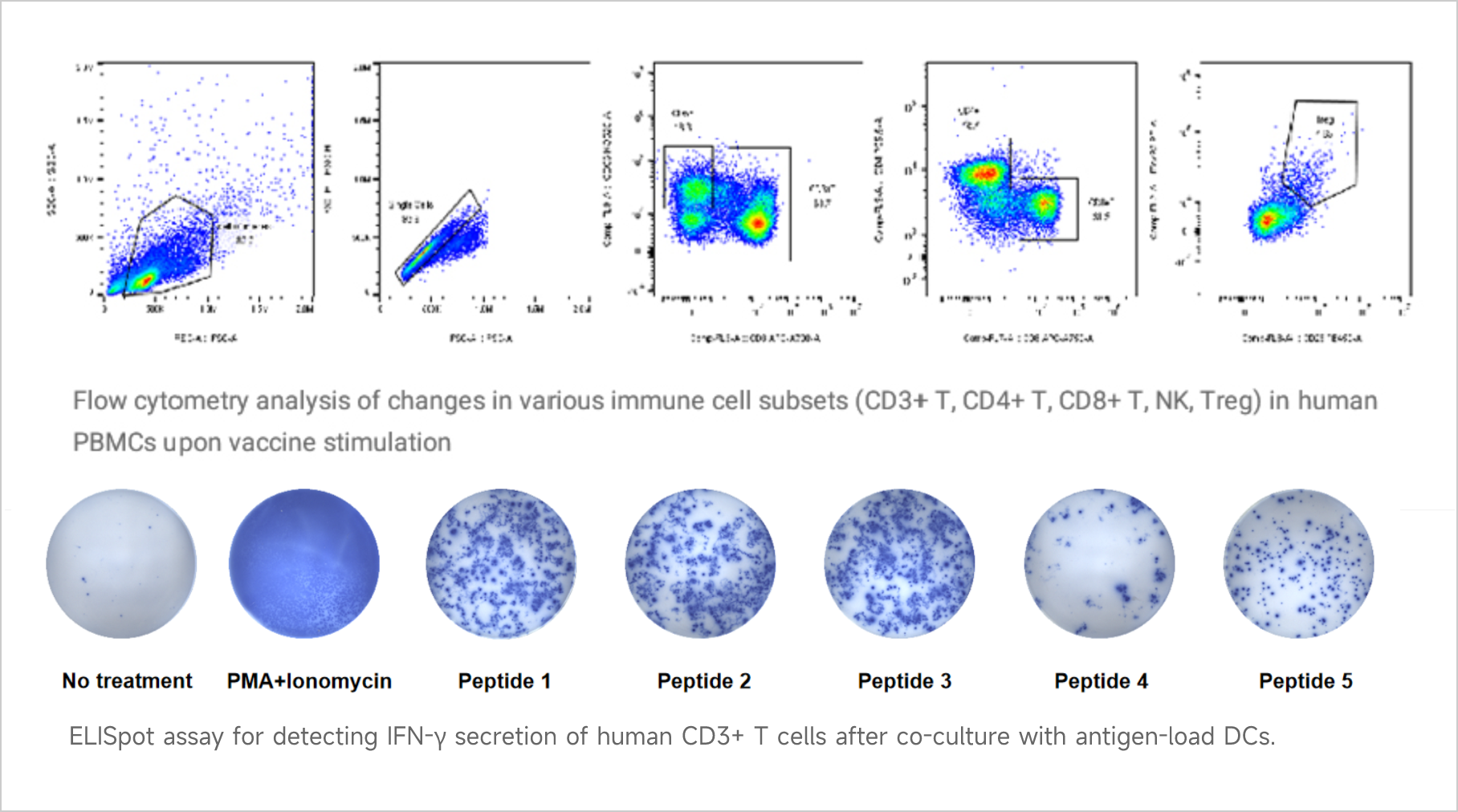
 Vaccine Immunogenicity Assessment
Vaccine Immunogenicity AssessmentViva Biotech has established a robust technology platform to provide preclinical vaccine immunogenicity assessment services to our clients. Among them are a variety of assays to evaluate T cell immunogenicity induced by different types of vaccines (e.g., mRNA vaccines, peptide vaccines, etc.) through co-culture of dendritic cells with T cells/PBMCs. These assays include, but are not limited to, FACS-based analysis of T cell subset changes and ELISPOT-based cytokine secretion assays.
Data Presentation
-
Other Functional Verification and Customized Experiments for Biologics
Viva Biotech offers a comprehensive suite of functional assays to characterize the immunomodulatory properties of antibody therapeutics for our clients, with customized experimental services available upon request.
Extracellular Cytokine Secretion Assay
Data Presentation