- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


LAGUNA HILLS, CA, November 07, 2020 – Arthrosi Therapeutics, a clinical-stage biopharmaceutical company focused on developing a best-in-class treatment for gout, has completed a Phase 2a clinical trial for company’s lead asset, AR882. Results from two clinical studies of AR882 conducted at Australia and New Zealand are being presented in Annual meeting of American College of Rheumatology Convergence 2020. AR882 demonstrated a strong safety profile, favorable pharmacokinetics, pharmacodynamics, and efficacy outcome in healthy volunteers and in gout patients.
Patients with chronic gout (baseline 9.0 mg/dL) were treated with AR882 50 mg in the phase 2a study, 95% of patients showed serum urate (sUA) level below 6 mg/dL and 5 mg/dL throughout the entire day. The results indicating AR882 considerably outperformed the current frontline therapies for gout and has potential to provide clinical benefit in hyperuricemia and gout patients. A phase 2b study for AR882 is current being planned. Arthrosi’s oncology candidate will soon be starting IND enabling studies with plans to submit the US IND at H2 2021.
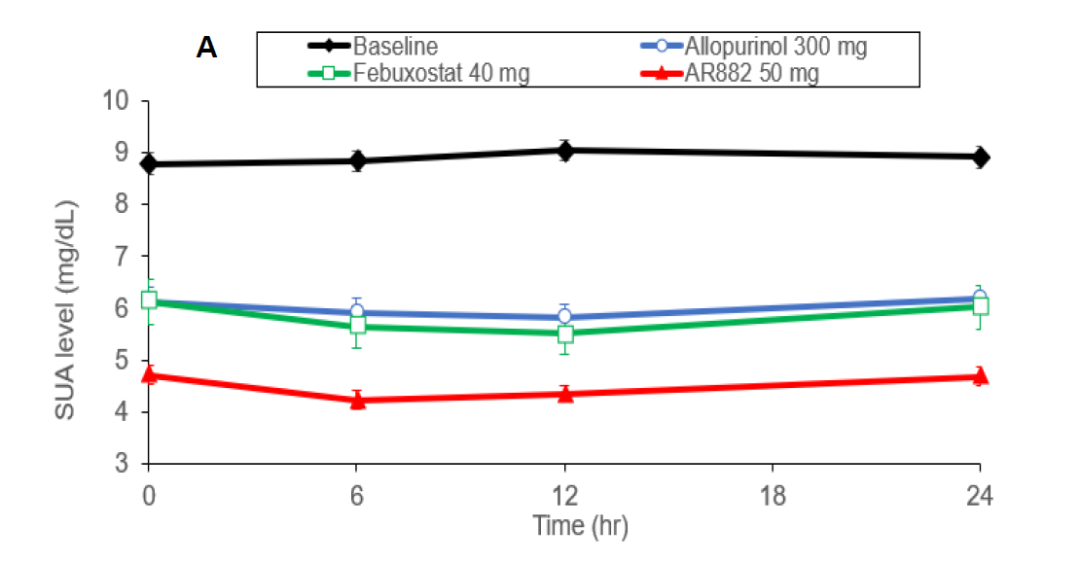
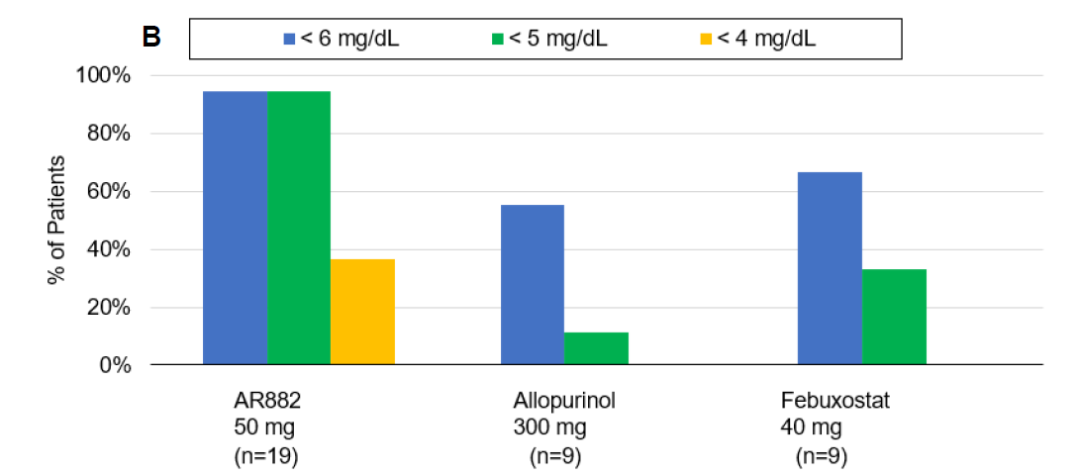
“Gout has a huge market. In China, there are 200 million hyperuricemia patients and nearly 20 million gout patients while the United States has 120 million hyperuricemia patients and 12 million gout patients,” said Litain Yeh, Arthrosi’s CEO," At Arthrosi, we are committed to the independent development of new drugs to address the unmet medical needs in the treatment for gout. We expect our drug to be the most effective and safe treatment to reduce the frequency of gout attacks and dissolve tophi. Eventually, Arthrosi’s treatment will become the first-line medication for gout treatment.”
“Our leadership team is comprised of key functional experts who have tremendous experience with the FDA’s drug approval process,” continued Dr. Yeh, “We have a range of successful preclinical and clinical trials as well as global approvals already under our belts. The team consists of the core scientists who did the research, management, and development of Lesinurad.
Dr. David Xu, Viva Biotech’s CBO and the leader of VBI, said,” Gout has a huge unmet clinical demand and market potential worldwide. We are excited by the progress and early data Arthrosi Therapeutics achieved in the clinical phase. As an early investor of Arthrosi, VBI has participated in three rounds of Financing, including the Series A, B and C, and will continue to assist in the research and development for AR 882 which is a new generation gout drug.”
Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us