- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


Home / News / Events / Events detail
The First Chinese biopharmaceutical Association CBA-China Annual Conference (CBA), was recently held at the Suzhou International Expo Center. Viva Biotech, a leading one-stop CRO/CDMO service company, was invited to participate in this conference. Viva Biotech attracted numerous industry professionals for in-depth discussions and consultations at booth, where the business team warmly welcomed visitors and provided detailed introductions to the company's extensive experience and unique insights in drug development and manufacturing.
Attendees showed strong interest in Viva Biotech's XDC technology and large molecule services. Addressing these key topics, the Viva Biotech team offered comprehensive explanations, emphasizing the company's technical advantages and successful case studies in these areas.

AI-Driven XDC Drug Discovery
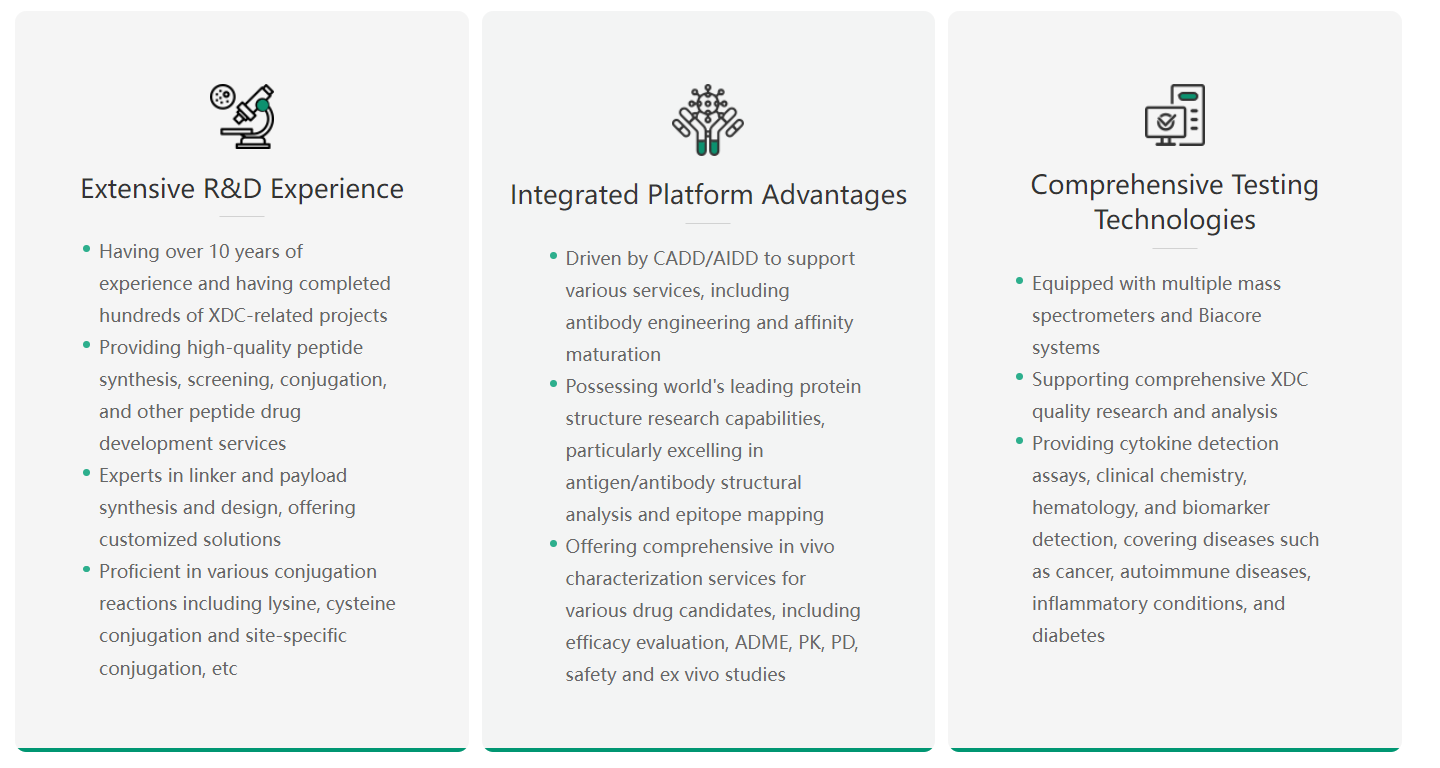
Viva Biotech leverages AIDD/CADD technologies to optimize drug design, significantly enhancing druggability and efficacy. The business team elaborated on optimizing linker-toxin molecule properties, designing ideal conjugation sites and affinity linkage strategies, and performing comprehensive antibody molecule scans to predict site-specific characteristics for precise ADC design. Additionally, Viva Biotech showcased its exceptional capabilities in peptide drug design optimization, structural analysis, and functional prediction.
AI-Enhanced Large Molecule/Antibody Drug Discovery

Focusing on structure-based drug discovery (SBDD) and integrating AIDD/CADD technologies, Viva Biotech has amassed extensive practical experience in antibody discovery, humanization, and affinity maturation. Several of the company's projects have advanced to clinical trial stages. Furthermore, Viva Biotech has obtained authorization for Lonza's bYlok® bispecific antibody pairing technology, bolstering its capabilities in bispecific antibody development.
Throughout the exhibition, Viva Biotech's booth attracted numerous attendees, generating positive feedback. This event not only showcased Viva Biotech's technical and innovative capabilities but also deepened collaborations with industry peers. Viva Biotech looks forward to future engagements with both new and old friends, further expanding the landscape of biopharmaceutical innovation.
Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us




