Home / News / Events / Events detail
In July, Viva Biotech attended several important events, including the 2023 Life and Healthy Industry Promotional Conference and 7th International Biomedical (Hangzhou) Innovation Summit at China (Zhejiang) Pilot Free Trade Zone, The 4th Global Conference & Exhibition on Biopharma Frontier Technology, Zhang Rui's Talk show | How CXO companies can break through in an era of fierce competition? 2023 China Chemical Drug Industrial Development Conference, the 2023 China Pharmaceutical Researchers Conference, and the SAPA-GP 2023 @Philly Cell and Gene Therapy Annual Meeting, among others. During these gatherings, Viva featured specialized booths to facilitate meaningful exchanges and collaborations. Attendees were privileged to witness captivating content and gain valuable insights from experts in the field.
The 2023 Life and Healthy Industry Promotional Conference and 7th International Biomedical (Hangzhou) Innovation Summit at China (Zhejiang) Pilot Free Trade Zone
On July 6th- 7th, the "2023 Life and Healthy Industry Promotional Conference and 7th International Biomedical (Hangzhou) Innovation Summit at China (Zhejiang) Pilot Free Trade Zone" was grandly held in Hangzhou Qiantang New District. The theme of the conference was "Innovation, Integration, and Transformation.", the event featured the participation of 6 esteemed academicians, including Yigong Shi, Weihong Tan, Yuguo Zheng, Dawei Ma, Dan Zhang, and Genhong Cheng, along with many other top domestic experts in industrial policy research who conducted a series of thematic workshops.

Second from right: Dr. Han Dai, CIO of Viva Biotech and Head of Viva BioInnovator
Dr. Han Dai, CIO of Viva Biotech and Head of Viva BioInnovator, was invited as a guest to participate in a roundtable discussion. The focus of the discussion was to explore the future strategy and industrialization hotspots concerning small molecule innovative drug development. During the session, Dr. Dai emphasized the significance of cutting-edge technology as a crucial factor in investment layout. However, he also highlighted the importance of considering other factors comprehensively, including the market competition pattern, pending clinical needs, and the R&D process, to make well-informed decisions.
The 4th Global Conference & Exhibition on Biopharma Frontier Technology
The 4th Global Conference & Exhibition on Biopharma Frontier Technology, co-organized by Tonacea and the National Center of Technology Innovation for Biopharmaceuticals (NCTI), and co-directed by four national associations/societies, was held in Suzhou International Expo Center, Jiangsu, China. Dr. Jianhua Cai, Senior Vice President of Viva Biotech, was invited to attend the conference and delivered a speech on the topic of “Sharing of PROTAC New Ligand Development Technology”.

“The screening of novel E3 ligase ligands is imperative, with particular emphasis on developing highly specific E3 ligase ligands, as they hold significant clinical relevance.”
Unlike small molecule inhibitors, PROTAC drugs operate through a catalytic mechanism and do not require prolonged binding to the disease-causing target. This characteristic is expected to address the challenges of "undruggability" and "drug resistance" associated with traditional drugs. Dr. Cai introduced cutting-edge research and development related to PROTAC, highlighting that only a few E3 ligases, such as VHL, CRBN, IAPs, and MDM2, have been utilized in PROTAC design. However, drug resistance may arise in PROTAC drugs developed based on these existing E3 ligases. Hence, there is a necessity to screen for new E3 ligases, particularly high-potency ones, and develop new E3 ligands. The development of highly specific E3 ligase ligands holds significant clinical importance.
We have advanced affinity screening technology platforms, including ASMS, Crystal soaking, SPR, and TSA screening technologies, which can support the development of new-generation E3 ligase ligands or novel target protein binding molecule screening requirements. ASMS technology stands out due to its high throughput, label-free nature, flexible screening, and rapid method development capabilities. ASMS can be performed in solution, making it suitable for screening fragment libraries and large drug-like libraries. During the presentation, Dr. Cai highlighted a case involving ASMS and SPR screening of molecular glues, which was exclusively designed by Viva.
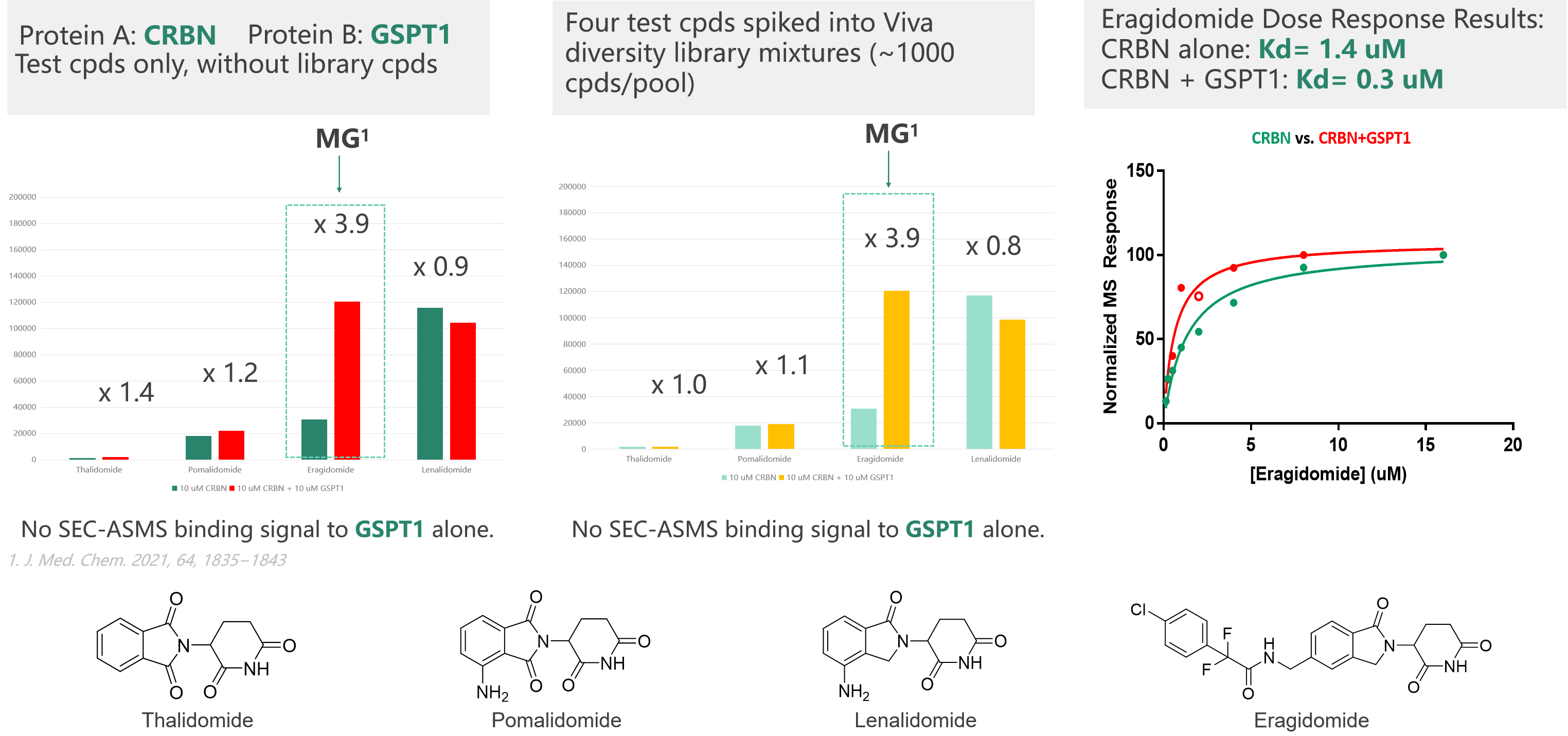
(Case study: SEC-ASMS Screening of MG)
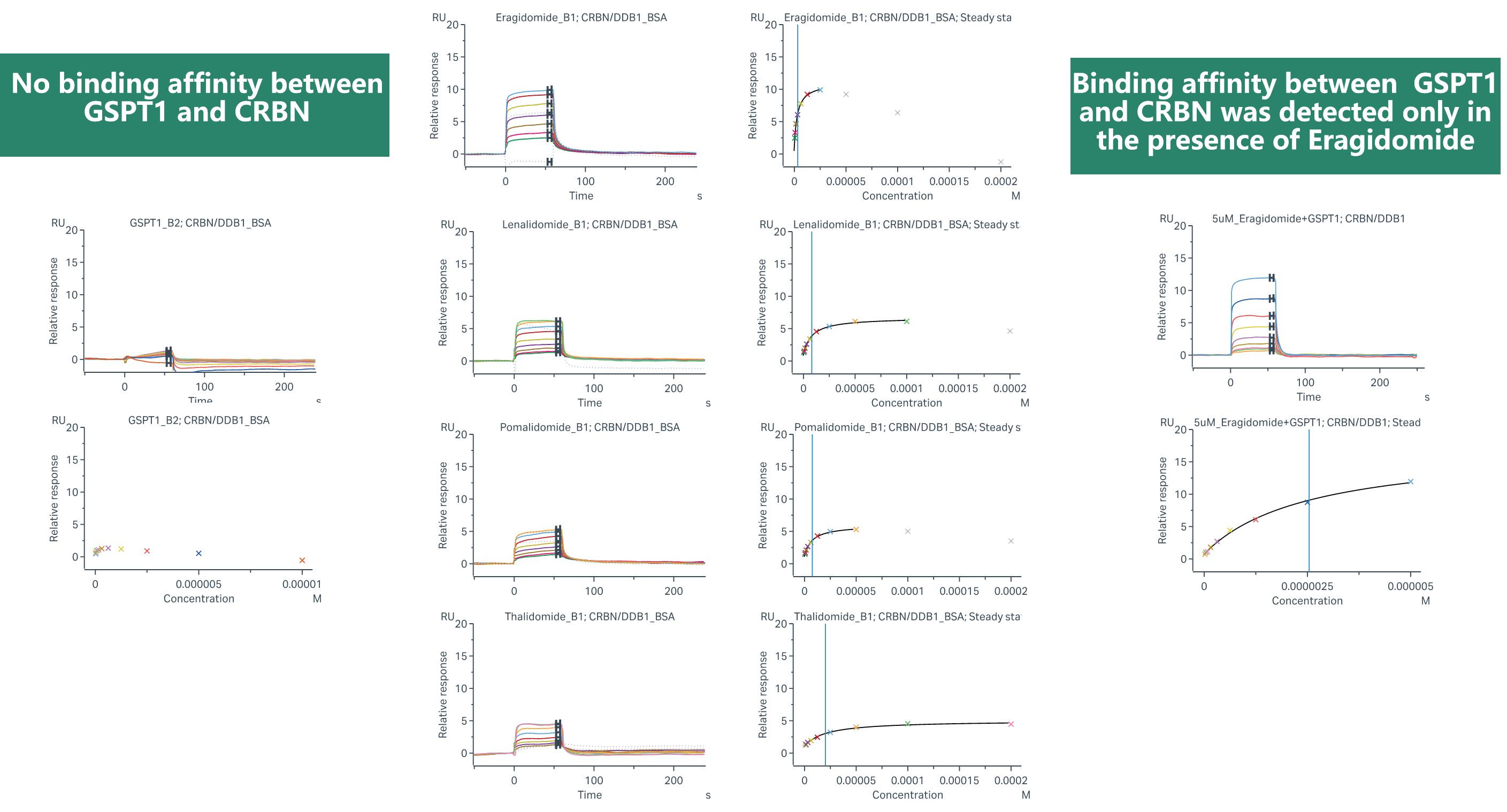
(Case Study: Binding Kinetics Study of MG with SPR)
In addition to the ligand screening technology, he also shared Viva's PROTAC platform, which covers a full range of biology and chemistry. By virtue of its strong protein structure research capability, Viva has accumulated more than 50 E3 ligases and delivered over 100 crystal structures of target protein-PROTAC-E3 ligase ternary complexes. In recent years, it has also established a method for the rapid analysis of CRBN ternary complex structures using Cryo-EM, with an average resolution of 3Å. Furthermore, Viva combines the Bioassay platform, chemical services, and DMPK to develop a mature drug discovery and development system. This system is complemented by CADD and AIDD to assist in the PROTAC molecular design and optimization process.
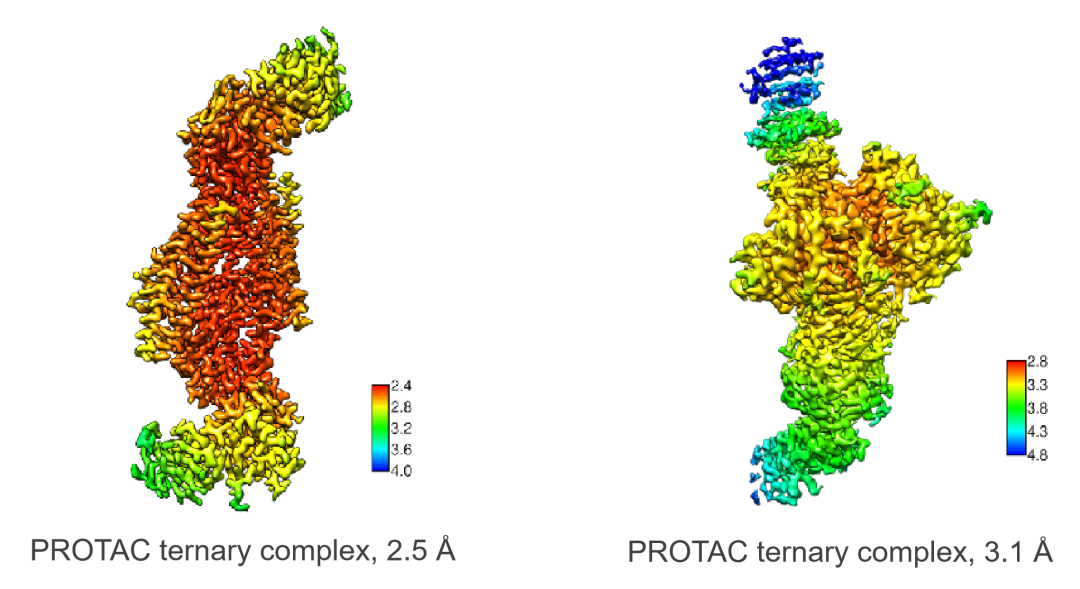
(Case study: PROTAC Structure Research with Cryo-EM)
At this conference, Viva also joined the “Tonacea Non-clinical Evaluation Alliance” and participated in the award ceremony as a member of the governing unit. The aim of the alliance is to bring together organizations with a vision and a sense of mission in the field of non-clinical evaluation in China. The goal is to explore new paths for drug development in China, promote the scientific formulation of non-clinical regulations and guidelines, cultivate more research, and development talents in the field of non-clinical evaluation, and improve the technical and management levels of new drug research and development in China. With the aim of embracing new opportunities and challenges brought about by changes in medicine, and collectively promoting the healthy development of new drug development, the conference will contribute to the advancement of new drug development in China.

During the exhibition, Viva's staff patiently received the visiting guests at the booth, shared with them Viva’s successful experience and professional insights in the field of new drug R&D and production, and fully demonstrated the one-stop comprehensive service capability, from early structure-based drug R&D to commercialized drug delivery.

Rui Zhang’s Talk Show | How CXO companies can break through in an era of fierce competition?
On July 13th, Dr. Xianyong Bu, Senior Vice President of Viva Biotech and Co-Founder&Managing Director of SYNthesis, was invited to " Rui Zhang’s Talk Show " organized by Healthcare Executive. Alongside several other industry experts, they engaged in discussions about the future challenges and opportunities for the development of the CXO industry.

"Looking at the global market, China's CRO still has a lot of room for development."
Regarding the question, "Has CXO entered the era of stock competition, Yes or No?" Dr. Bu provided insights from the perspective of CRO companies: Both answers, "Yes" and "No," hold merit. "Yes" is relevant because the global biopharma investment and financing market currently operate in a rationalized environment, leading to a reduction in outsourcing demand due to upstream budget cuts. On the other hand, "No" is also plausible as Dr. Bu presented his argument in two dimensions:
Firstly, considering the global market, China's CRO industry is far from being over-saturated. Public data reveals that by 2025, the global CRO market is anticipated to reach a volume of hundreds of billions of US dollars, whereas China's CRO market during the same period might be less than a quarter of that magnitude. Moreover, China's substantial domestic engineering dividends and low labor costs are not proportionate to this scale, suggesting considerable growth potential for the country's CRO industry.
Secondly, examining the past 4-5 years, China's CRO sector has indeed experienced a downward trend. Nevertheless, Dr. Bu emphasized that considering a more extended timeframe of 10-20 years, such fluctuations are natural stages of development.
2023 China Chemical Drug Industrial Development Conference
From July 13th to 14th, the 2023 China Chemical Drug Industrial Development Conference took place in Shanghai, with a primary focus on the biopharmaceutical sector. The summit gathered nearly a hundred renowned professionals from research, production, and investment fields, engaging in comprehensive discussions on the latest advancements and future directions in chemical drug research and development. Covering aspects like strategy, market dynamics, regulations, innovative research, and production processes, the event aimed to boost industry growth through efficient resource integration. Among the distinguished attendees were Dr. Han Dai, CIO, Head of Viva BioInnovator, and Dr. Jianguo Ma, Senior Vice President of Viva Biotech and CEO of Langhua Pharmaceutical, who played key roles as the host and keynote speaker, respectively.

First from left: Dr. Han Dai, CIO, Head of Viva BioInnovator
Does the current trend in small molecule innovative drug research and development adequately meet the future demands of medicine? For instance, is it geared towards highly personalized and tailor-made treatments for the future? Dr. Dai participated in a roundtable discussion with the attendees, where they exchanged ideas and explored the present market demands and potential future growth of small molecule innovative drugs.

"Biosynthesis technology will be an important development direction for green pharmaceuticals."
Currently, "green pharmaceuticals" have become pivotal for pharmaceutical companies striving to achieve their dual carbon goals. Innovating key technologies stands as a crucial method in streamlining manufacturing processes. During one of the forums, Dr. Ma delved into the application of green technologies in the pharmaceutical industry, saying that in the future, various cutting-edge technologies, such as continuous flow reactors, enzyme catalysis, high-throughput screening platforms for metal-catalyzed/synthetic reactions, photocatalysis, wastewater/organic solvent treatment technologies, and aqueous-phase chemistry, will all play pivotal roles as key components in the realm of green pharmaceuticals. He highlighted that implementing green technologies not only bolsters a company's competitiveness but also serves as a significant contribution to environmental protection.
2023 China Pharmaceutical Researchers Conference
The 2023 China Pharmaceutical Researchers Conference took place in Shanghai on July 14-15. The event brought together domestic drug developers, innovators, and technology service partners, creating a platform for sharing cutting-edge experiences and the latest advancements through focused sub-forums. These sub-forums covered various crucial areas, including industry development trends, R&D hotspots, development strategies, improved new drug development, raw material, and formulation development, new drug initiation and clinical development, quality control, registration and declaration processes, MAH policies, and the investment and financing market environment.

Dr. Jianguo Ma, Senior Vice President of Viva Biotech and CEO of Langhua Pharmaceutical, delivered an engaging keynote speech titled "Innovative Drug API Development, Production, and Regulatory Approval from IND to NDA." During his talk, he provided valuable insights and shared his technical experiences, illustrating successful case studies of API development and production at Langhua Pharmaceutical, a subsidiary of Vial Biologics, across different clinical stages. Furthermore, he highlighted Langhua Pharmaceutical's capabilities, strengths, and achievements in offering comprehensive CMC (Chemistry, Manufacturing, and Controls) services. This encompassed a top-notch research and development team, state-of-the-art platforms for raw material and formulation technologies, ample production capacity, reliable GMP and EHS management systems, and a robust IP protection framework. Leveraging these advantages, Langhua Pharmaceutical efficiently and flexibly caters to customers' comprehensive CDMO (Contract Development and Manufacturing Organization) needs for small molecule drug research and development, ensuring efficient, flexible, and high-quality services.
SAPA-GP 2023 @Philly Cell and Gene Therapy Annual Meeting
From June 23rd to 24th, the highly anticipated SAPA-GP 2023 @Philly Cell and Gene Therapy Annual Meeting was proudly hosted by the Sino-American Pharmaceutical Professionals Association — Greater Philadelphia (SAPA-GP). The primary objective of this conference was to provide a dynamic face-to-face platform for technological innovators, industry leaders, supply chain vendors, government officials, and high-tech investors in the biopharmaceutical realm. The focal point of the event centered around cell and gene therapy, fostering valuable exchanges and sharing of entrepreneurial experiences within this cutting-edge field.

Dr. Cynthia Cai, the Business Partner at Viva BioInnovator, was invited to participate in the panel discussion on "The Journey of Growth for Biotech Founders/CEOs." Alongside other esteemed guests, she engaged in insightful discussions about the essential aspects for entrepreneurs, spanning from ideation and startup to fundraising, intellectual property protection, and effective board governance.
Furthermore, she took charge of moderating the roundtable session titled "Fostering Synergy and Growth between the Board and CEO," where she delved into the inner workings of boards in early-stage biotech companies. During this session, she shared practical experiences of successful collaborations between CEOs and board members.

