Home / News / Events / Events detail
On October 29, 2021: Viva BioInnovator (VBI) successfully hosted its 4th online Demo Day. Six companies from among VBI's 80+ portfolio companies presented their cutting edge technology: Evecxia Therapeutics, Basking Biosciences, Mebias Discovery, AcuraStem, VersaPeutics and Genhouse Bio. These companies shared their latest developments with indications covering depression, stroke, ALS, oncology, and more.
Nearly 200 top-tier investors and pharma reps attended the event, with the majority of attendees coming from China. These attendees participated in Q&A sessions with the company founders that explored their market potential and scientific progress. After each company's presentation, Associates from Viva BioInnovator shared investment highlights covering technical characteristics, market prospects, and competitive advantages.

After the roadshow session, Dr. Amanda Fu, CEO of Redbud Medicine, Dr. Yongjiang Hei, Co-CEO of Wuxi Biocity, Dr. Haichen Yang, Vice President of Clinical Research of Amicus Therapeutics, and Dr. Han Dai, Chief Business Officer of Viva Biotech and Head of Viva BioInnovator, had an in-depth discussion on Making the Jump: How to Prepare for the Clinic.
On the question of whether to hire an internal team or choose a CRO to conduct clinical trials, Dr. Haichen Yang, Vice President of Clinical Research of Amicus Therapeutics, said, "Small companies often choose to partner with a CRO when they do not have a large team. When choosing this option, it is essential to choose international CROs with extensive experience in clinical development. Their strong technology platform and team can help you to achieve your goals quickly. However, as your company moves into the next phase of development, you still need to eventually develop your own internal team and build a unique technology platform."
Regarding the location for conducting clinical trials (Australia, China, U.S., global), Dr. Amanda Fu, CEO of Redbud Medicine, said, "Phase 1 clinical trials on healthy subjects in Australia are progressing quite fast, but for clinical trials on cancer patients, the enrollment is much slower. So we have to choose the trial location based on the clinical subjects (healthy population or cancer patients). In addition, we have to consider the different requirements for filing an IND in the U.S. and ensure a smooth transition for clinical trials conducted in different countries. With the globalization of new drug development, multi-regional clinical trials (MRCT) have been widely adopted for new drug applications in different countries. Currently, MRCTs can be conducted simultaneously in the U.S. and Australia."
On the selection of biomarkers for patient grouping, Dr. Yongjiang Hei, Co-CEO of Wuxi Biocity, said, "For each area of disease research, biomarkers can be used to learn the biological process the organism is undergoing. Examining a disease-specific biomarker can be useful for disease identification, early diagnosis, prevention, and monitoring during treatment. For example, clinical predictions can be made by using biomarker measurement in phase 1 clinical trials."
Dr. Han Dai, Chief Business Officer of Viva Biotech and Head of Viva BioInnovator, concluded, "For small and medium-sized biopharmaceutical companies, internal management is important, having the right professionals in key positions. In addition, we need to find trustworthy external partners to assist in jointly advancing the clinical trials of new drug development. Moreover, we need to consider the company's strategy, pipeline layout, indications, and other factors to develop appropriate patient enrollment strategies to prepare for subsequent clinical trials."
If you missed the live stream, the following section covers highlights from the presentations given during the Demo Day.
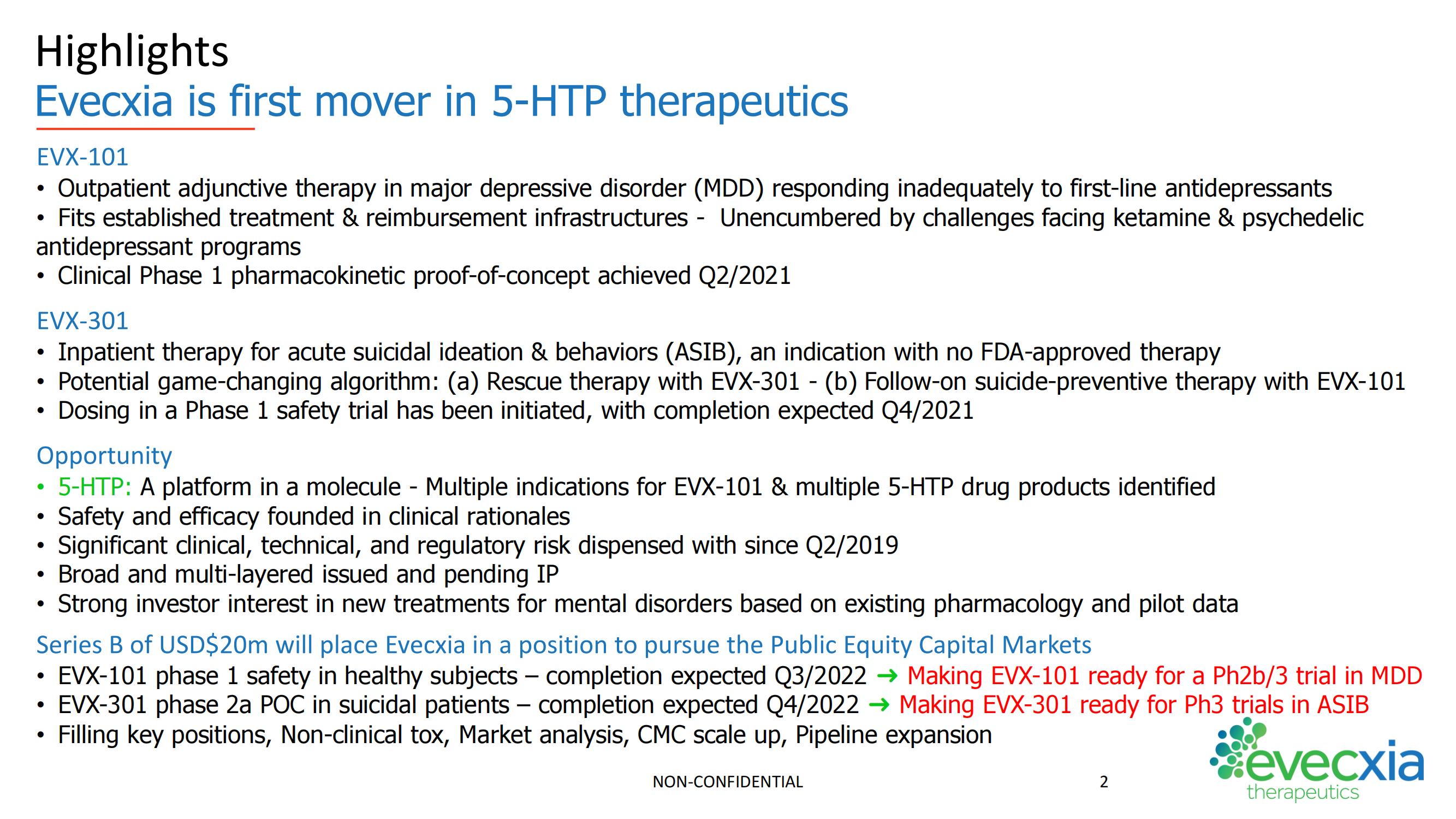
Evecxia Therapeutics (Financing round: Series B, USD)
Speaker: John Kaiser, Evecxia Therapeutics CEO
"Evecxia is the first company dedicated to realizing the therapeutic potential of 5-hydroxytryptophan (also known as 5-HTP), to treat mental illnesses. In particular, EVX-101 is an oral slow-release tablet of 5-HTP and low-dose carbidopa and is being developed as an adjunctive treatment for depression when first-line antidepressants alone are inadequate. EVX-301 is a proprietary, 24h, intravenous infusion of 5-HTP and is being developed as a rescue therapy in acute suicidal crisis. Evecxia's value proposition combines a de-risked approach to CNS drug development with novelty, differentiation, and multiple shots on goal."
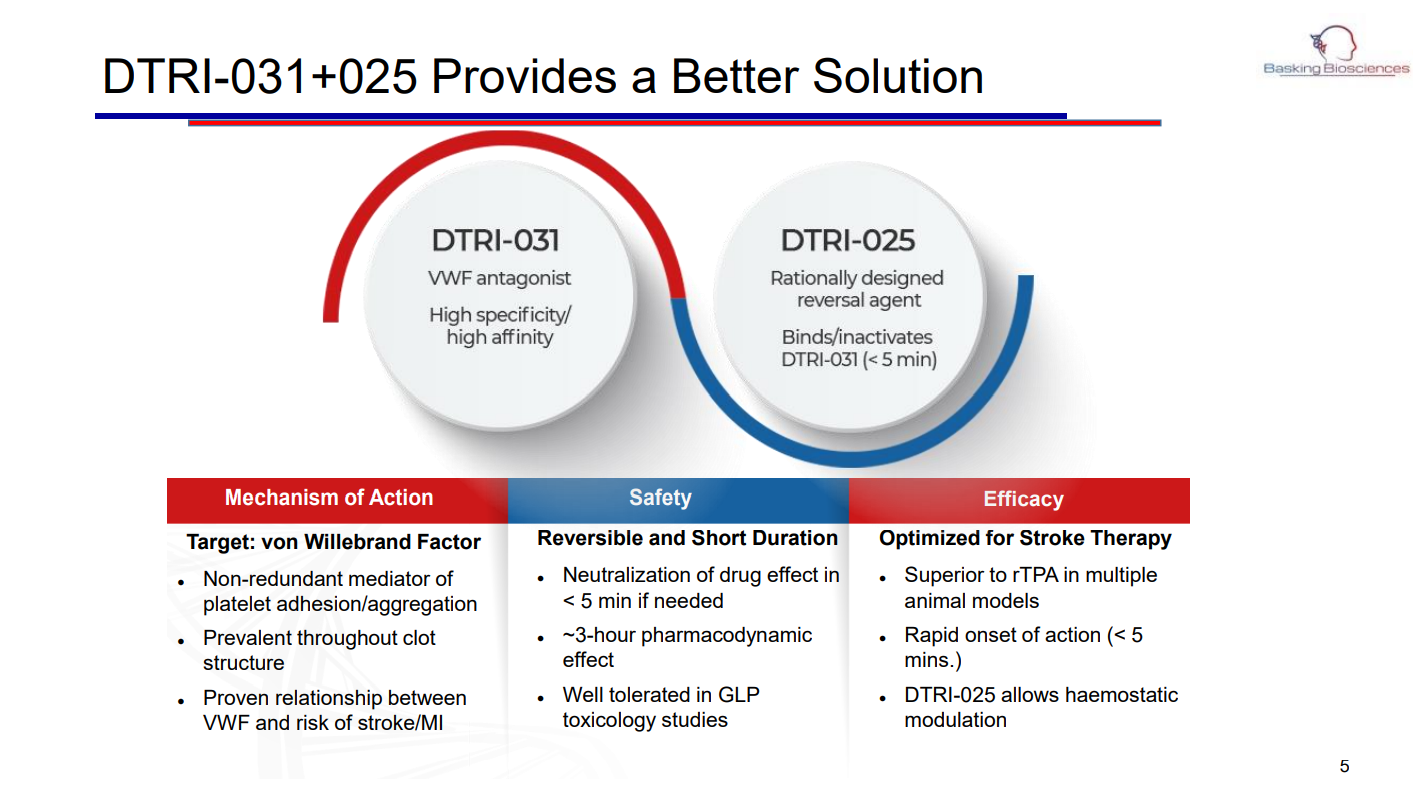
Basking Biosciences (Financing round: Series A, USD)
Speaker: Richard Shea, Basking Biosciences CEO
"Stroke is an area of high unmet medical need, the second most deadly disease worldwide, and one of the leading causes of severe long-term disability. 85% of strokes are ischemic, and the only intervention currently proven to improve long-term outcomes is the rapid restoration of blood supply to the brain. However, all currently available treatments have limitations, so only about 15% of patients receive an intervention. To address these existing problems, Basking is developing a reversible drug to restore blood supply to the brain in ischemic stroke patients. Basking's team has a world-leading advisory panel including the former head of the U.S. FDA, and the company has been granted an exclusive patent by Duke University."
Mebias Discovery (Financing round: Series B, USD)
Speaker: Shariff Bayoumy, Mebias Discovery Co-Founder
"Mebias is an emerging leader in drug discovery/development of compounds targeting G-protein coupled receptors (GPCR), enabling the accurate design of GPCR-targeted drugs with well-established mechanisms that can avoid specific side effects within a therapeutic index (called pathway-selective drug discovery). This provides a unique opportunity with lower risk than exploring new, unproven drug mechanisms using traditional techniques. The company plans to conduct a phase 1 clinical trial for a new preclinical Mu opioid candidate in 2022. It is noteworthy that this is an oral, non-addictive analgesic and is also an NIH-supported research program."

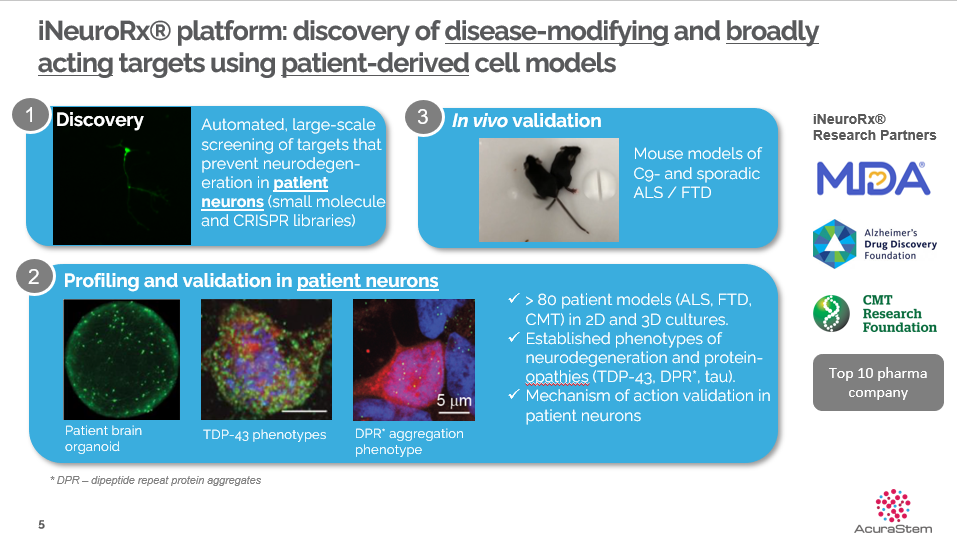
AcuraStem (Financing round: Series A, USD)
Speaker: Sam Alworth, AcuraStem CEO
"AcuraStem, a biotech company developing treatments for neurodegenerative diseases, has built a unique technology platform based on neurons derived from sporadic ALS patients. This platform is well-positioned to reproduce disease symptoms driven by unknown genetic causes, helping the company complete the discovery of mechanisms and validation of MOA to find new therapies that may improve patient conditions. AcuraStem is developing therapies that offer significant advantages over other drug candidates in preclinical development based on this platform. The company expects to apply therapies validated in multiple patient samples in vitro to patients with sporadic ALS in the clinical setting."
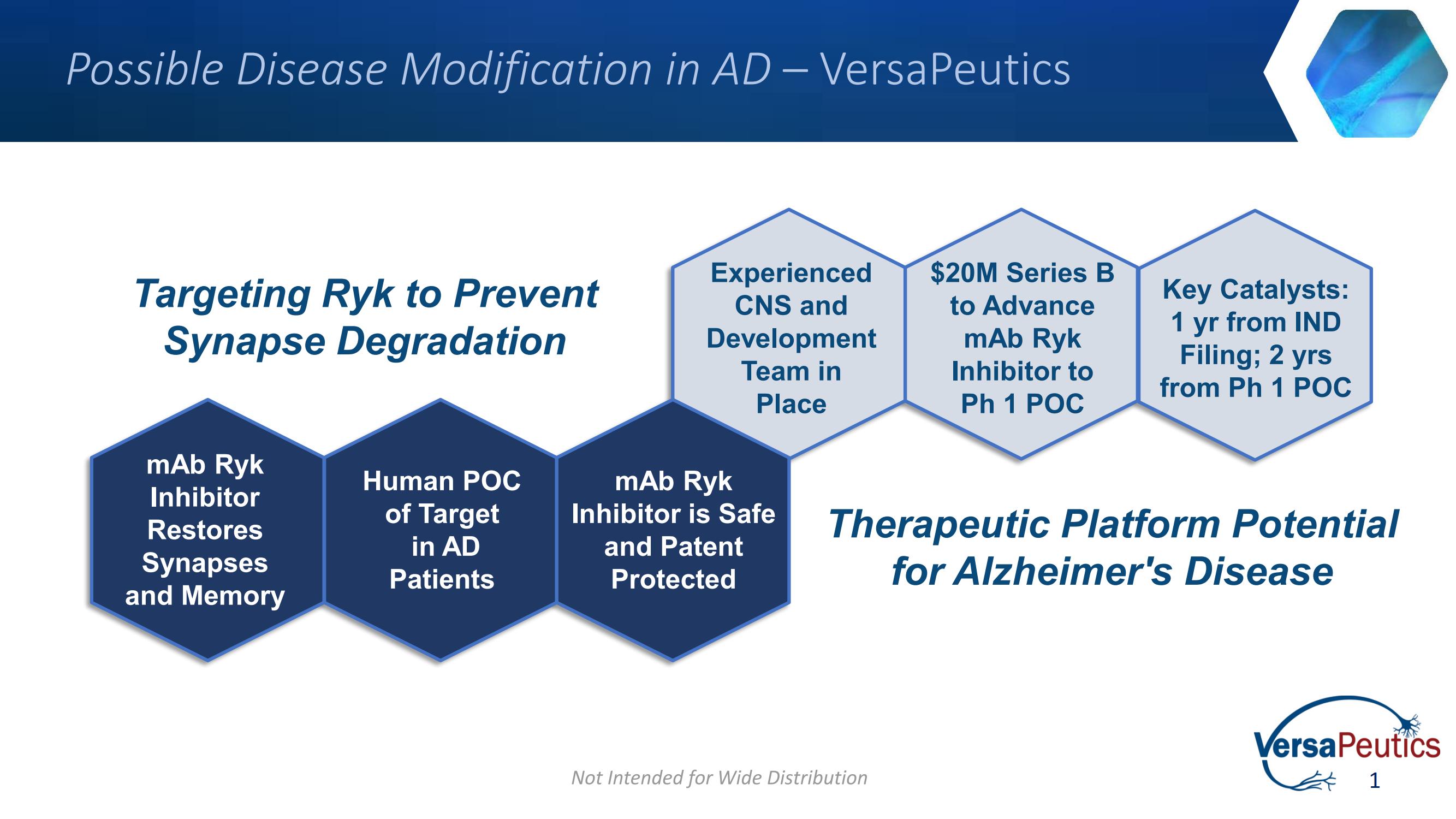
VersaPeutics (Financing round: Series B, USD)
Speaker: Chad Beyer, Ph.D., MBA, VersaPeutics SVP
"VersaPeutics is a biotech startup focused on regulating the Wnt/PCP signaling pathway for the treatment of Alzheimer's disease, as well as developing treatments for spinal cord injury, neuropathic pain, and oncology. Its monoclonal antibody against Ryk. VMab-101 demonstrated robust protection against amyloid-beta-induced synaptic degradation and has been shown to restore memory and cognitive performance in preclinical models."
Genhouse Bio (Financing round: Series A+, RMB)
Speaker: Kuifeng Wang, Ph.D., GenHouse Bio Founder and CEO
"Genhouse Bio is a biotech company focusing on the development of next-generation anti-cancer therapeutics with leading small molecule drugs. The company's management team has years of experience in corporate management, new drug development, and clinical research. Genhouse Bio has built a highly innovative anti-cancer pipeline based on its integrated drug development platform and moved its leading candidate, KRAS G12C inhibitor GH35, into a Phase I clinical study. In addition, another drug candidate, SHP2 inhibitor GH21, has been approved in the U.S. With more than 30% of the pathways in tumors associated with it, this candidate has high international value and a great future market potential."
With the mission of "building an open platform for global biopharmaceutical startups," VBI continues to promote and develop a pharmaceutical innovation ecosystem, hoping to establish an effective network with extensive cooperation and the ability to achieve a win-win situation for all parties. VBI Demo Day was born with this mission, and we hope that more founders and investors can conduct efficient and in-depth communication and achieve mutual success through future Demo Days. If you are interested in further contact with the presenting companies, please send an email to innovation@vivabiotech.com and we will get back to you as soon as possible.

