- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


Home / News / Events / Events detail
Zhangjiang Pharma Valley Online Courses - Affinity-Based Drug Screening Technology
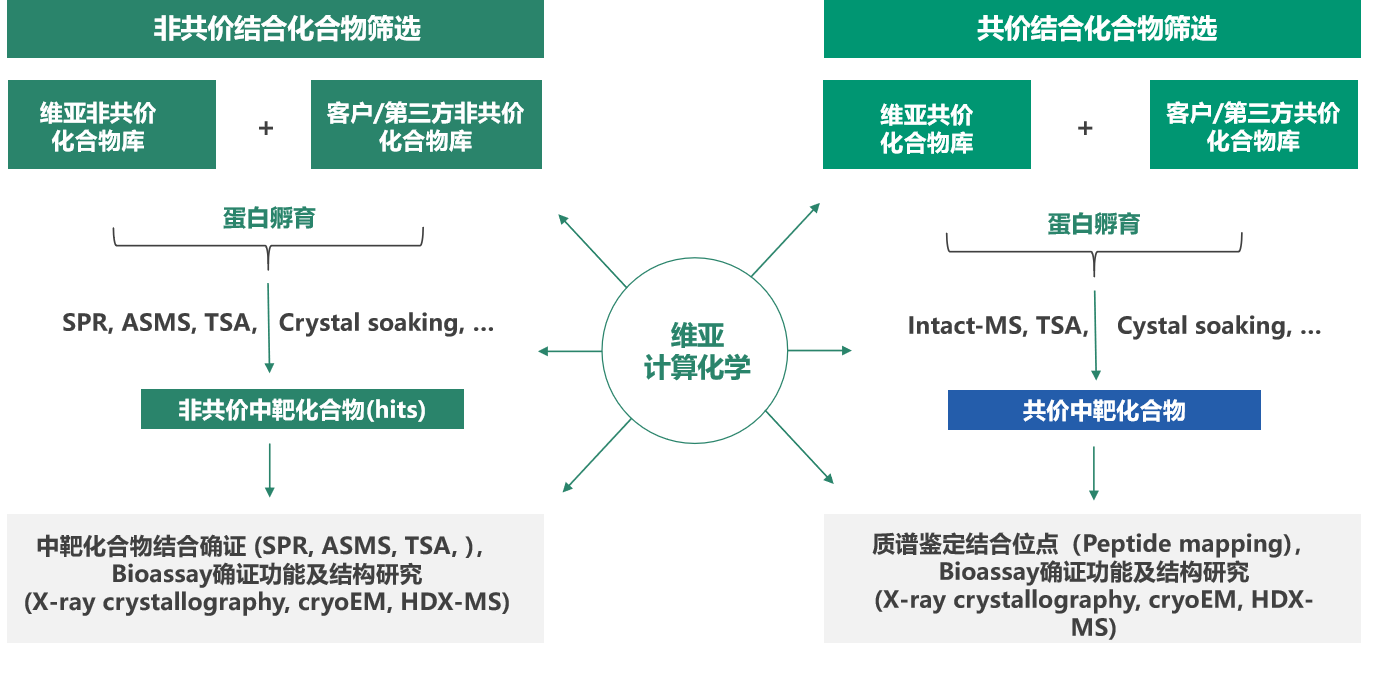
On May 26th, Dr. Jianhua Cai, Senior Vice President of Viva Biotech, give a lecture on "Affinity-based Drug Screening Technology" at Zhangjiang Pharma Valley Online Courses. He introduced the common affinity screening methods and highlighted the affinity screening platform of Viva. He introduced the current affinity-based screening technologies for non-covalent and covalent compounds, and introduced the application of TSA, Crystal soaking, SPR, ASMS and Intact-MS through case study. TSA and Crystal soaking can screen both non-covalent and covalent library, SPR and ASMS screen non-covalent library and Intact-MS screen covalent library.
In addition, Viva has several libraries: Viva Fragment Compound library-1 with 2500 compounds mainly for ASMS; Viva Fragment Compound library-2 with 960 compounds mainly for SPR and X-ray Crystal Structure Analysis. Viva has a GPCR related compound library of 6500 compounds and large diversity compound library of 200000 compounds. Viva also have a series of advanced screening technologies, which can screen the Viva compound library and the clients’ compound library .
Finally, Dr. Cai concluded that "Different screening methods correspond to respective uses, and are applied and validated with multiple methods during the drug discovery phase. We need to choose the appropriate screening method for respective projects."
VB-NeoBio Online Panel - Protein Degraders
On May 26th, VB-NeoBio and Med-Fine Capital held a online panel on "How Protein Degraders Will Continue to Evolve in the Next 20 Years?" Dr. Han Dai, CIO of Viva Biotech and Head of Viva Bioinnovator, joined as one of the panelists to share his insights.
Dr. Han Dai shared the limitations and opportunities of breaking through the mature targets of protein degradation technology, and combined experience and technical advantages of research and development of Viva Biotech to give an in-depth explanation.
On the topic of "Difficulties to be broken through in protein degradation technology at present", he emphasized, "Although the advantage of PROTAC is to difficult drug targets, the early work is still on validated targets. Due to the physicochemical characteristics of the protein degradation molecule, its druggability have been challenged in the past years. With the accumulation of experience, protein degradation-related technologies such as molecular design, physicochemical characteristics prediction, and formulation research, etc. have been greatly improved. So we believe that there is great potential in this field, which makes it possible to solve problems that cannot be solved by traditional small molecule drugs."
CBIITA Alliance Online Panel - Innovation and Challenges in the Global Biopharmaceutical Industry
On May 18th, "Innovation and Challenges in the Global Biopharmaceutical Industry" Panel was held online by CBIITA Alliance. Dr. Han Dai, CIO of Viva Biotech and Head of Viva Bioinnovator, joined as one of the panelists to share his insights.
On the topic of "For pharmaceutical companies, the choice of License in or License out in licensing partnership deals", Dr. Han Dai said: "In recent years, many Biotech companies have focused on the field of innovative drugs, and many domestic traditional pharmaceutical companies have transformed into innovative drugs. Whether it is License in or License out, the strategies of the company and its partners should be considered and synergize with each other so that achieve a win-win situation."
Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us