The antibody drug discovery process is a long journey requiring substantial time, effort, and financial investment, with only a mere 5% of candidates making it to the market. However, the advancement of Artificial Intelligence Drug Discovery (AIDD)/ Computer-aided Drug Discovery (CADD) technology may bring a turning point to this arduous process. How will the fusion of artificial intelligence (AI) and biotechnology transform the antibody-drug R&D process?
Viva Biotech is making significant strides in this cutting-edge field, exploring new directions such as generative AI-driven de novo design of antibodies. Once the initial antibody sequence is identified, Viva's research team utilizes advanced structural biology knowledge and AI technologies to humanize or animalize antibodies, ensuring their safety and efficacy. Additionally, they have developed a highly automated workflow for antibody affinity maturation, further optimizing antibody performance and specificity. This innovative approach combines AI-driven drug design (AIDD/CADD) with structural biology and rigorous experimental validation, reshaping the standard processes in antibody drug discovery.
Core technology platforms
Phage display technology platform, hybridoma platform (mouse monoclonal antibodies, rabbit monoclonal antibodies), nanobody discovery platform (alpaca antibodies, camel antibodies)
Shorten R&D cycle time by 50%
By modifying existing antibody sequences and utilizing de novo design, the antibody discovery time can be shortened from the traditional 6-8 months to 3-4 months, significantly reducing R&D costs and increasing success rates.
Over 10+ years of antibody/biologics R&D experience
Integrating multiple leading technologies, offering customized, one-stop services from antibody discovery to cell line development.
SBDD-based, AIDD/CADD-driven
Leveraging Viva Biotech’s globally leading protein structure analysis platform and powerful AIDD/CADD platform to support antibody R&D.
Case study: Antibody discovery
Assisted by AIDD/CADD, the Viva Biotech R&D team successfully completed the entire process for a client, starting from antigen design and expression to fully human antibody screening and subsequent affinity maturation. By analyzing the structure of the antigen-antibody complex, they discovered that the antibody binds to the antigen at a unique angle of approximately 45 degrees, distinguishing it from other competing products. Despite the primary sequence of the antigen epitope being slightly different, this 3D structural difference explained the distinct mode of action: the antibody selectively binds to the antigen on tumors without binding to antigens on red blood cells, thus reducing side effects. This discovery opened the door to additional research, ultimately leading to a significant licensing agreement. The project is currently in Phase II clinical trials.
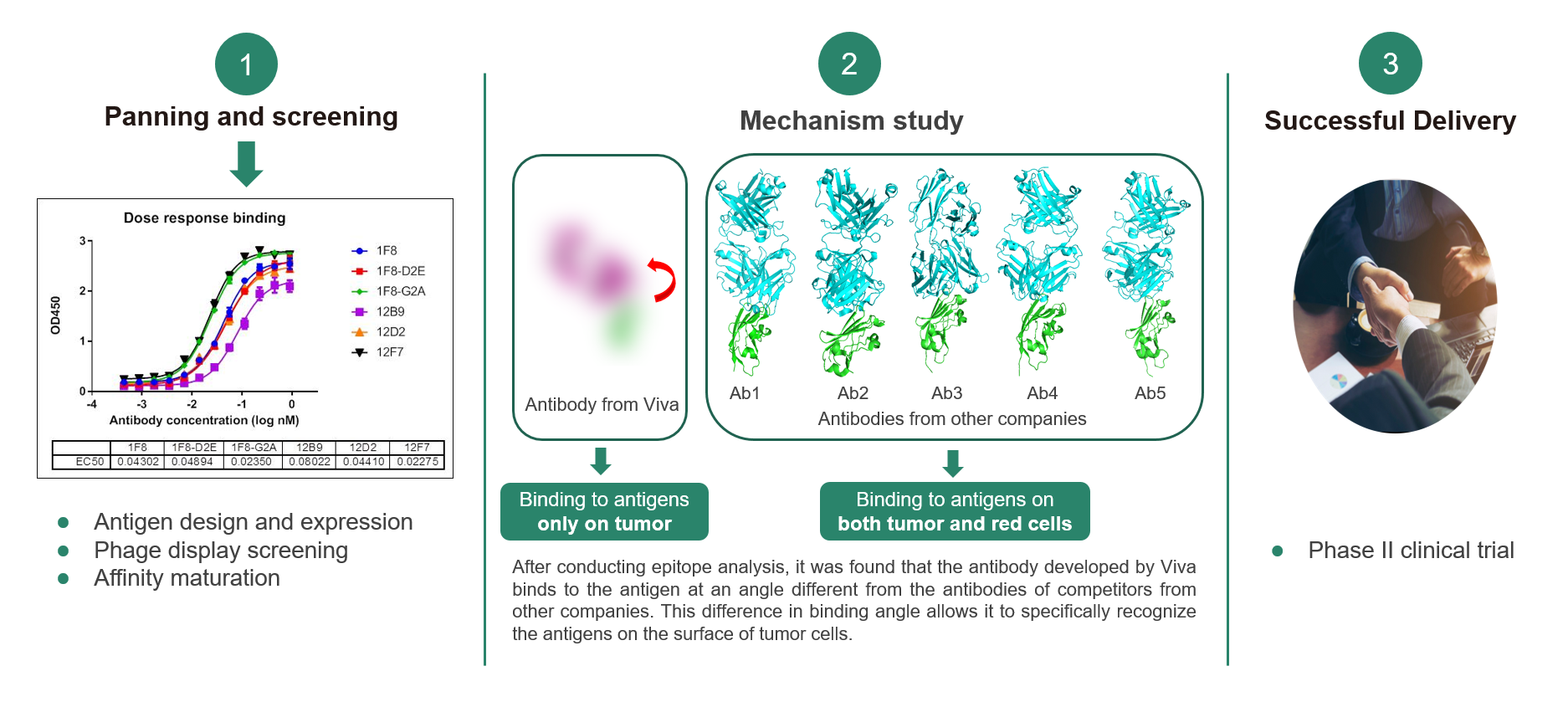
Case study: Antibody humanization
Researchers utilized CDR region transplantation and AI-assisted design strategies to precisely achieve humanization of antibody sequences, minimizing immunogenicity while maximizing the retention of antibody affinity and other key molecular properties. In the left case, an antibody derived from rodents was successfully humanized through CDR region transplantation and design optimization, with the affinity of the antibody remaining comparable to the original molecule. The middle case shows a similar outcome, with the humanized antibody maintaining nearly the same affinity as the original. Notably, in the case on the right, Viva Biotech not only successfully humanized the antibody but also improved the affinity of the humanized antibody achieving an unforeseen enhancement.
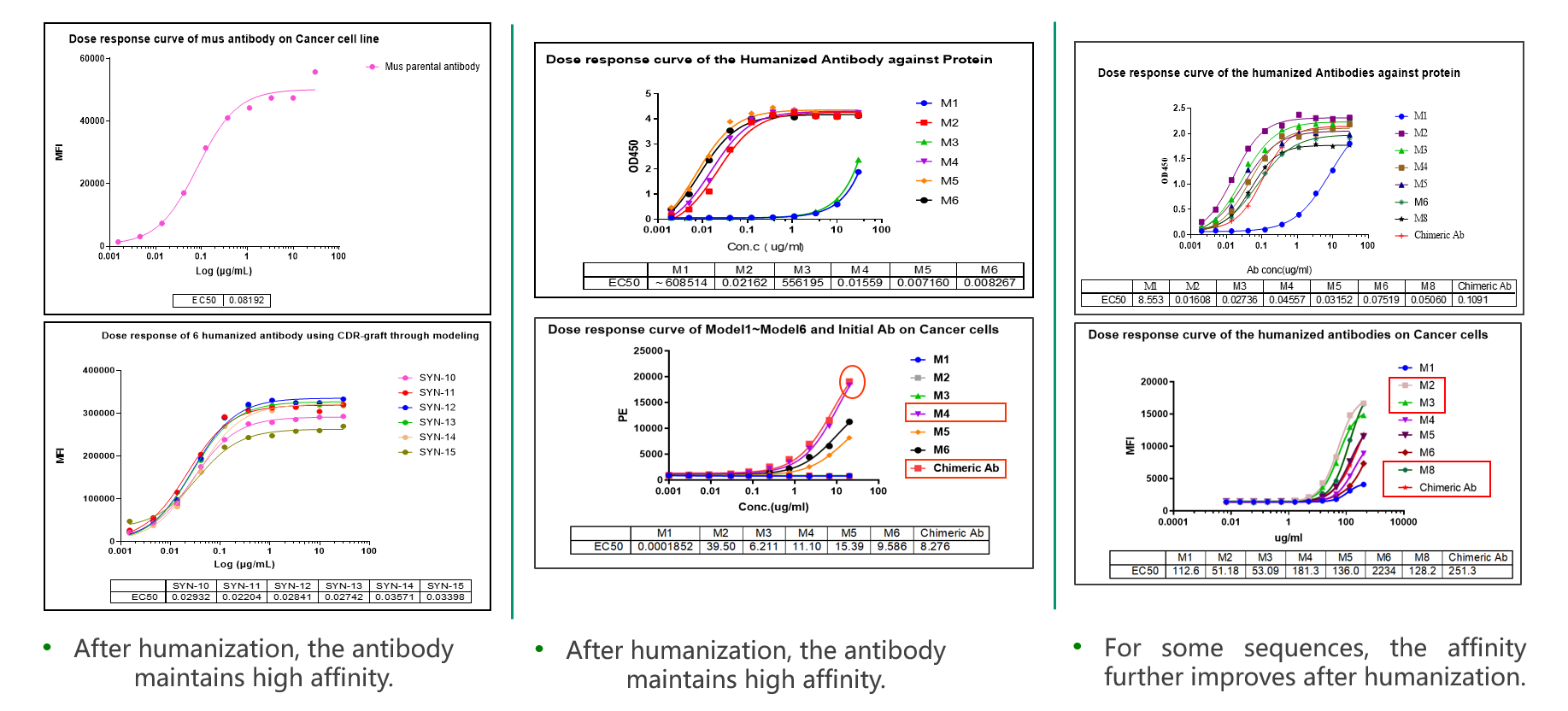
Case study: Antibody engineering
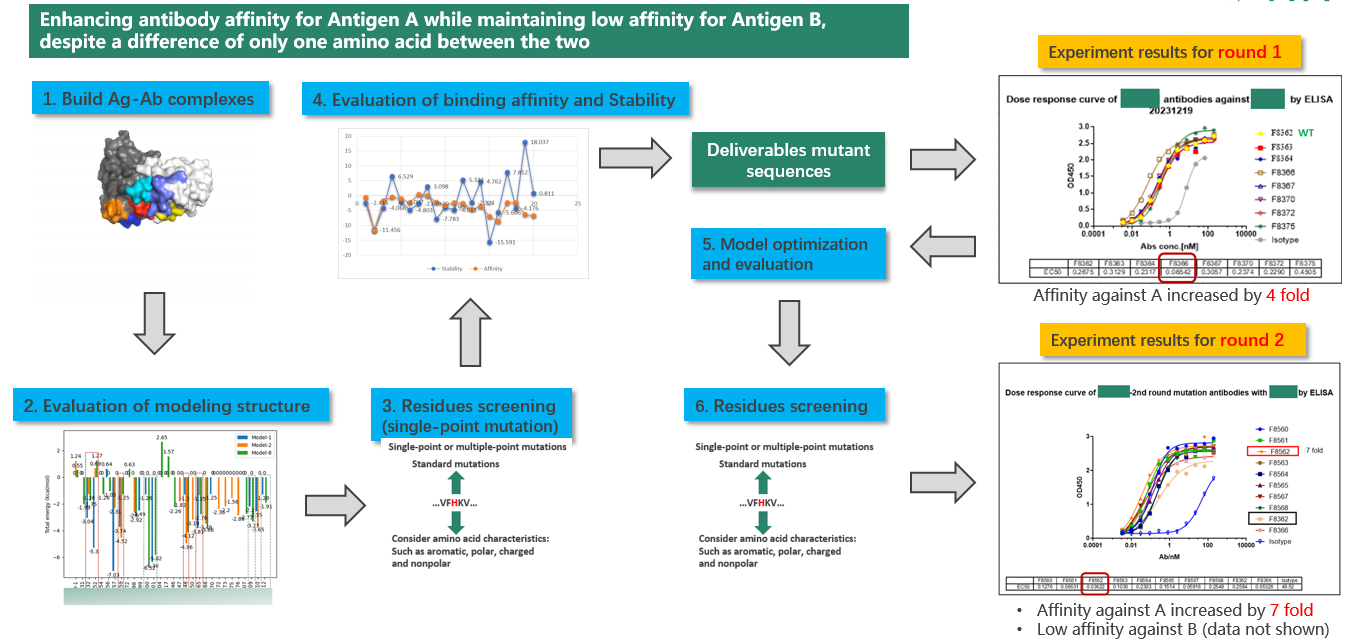
The client had two homologous proteins, Antigen A and Antigen B, and required an antibody with increased affinity for Antigen A while maintaining low affinity for Antigen B, presenting a significant challenge. The research team began by analyzing the structures of the antibody-antigen A/B complexes to understand their binding modes. Then, they utilized AI algorithms to simulate and assess the impact of amino acid mutations in the antibody's CDR regions on affinity, taking stability and other factors into account. Using heat maps to predict results. The team combined crystal structure analysis, AI predictions, and high-throughput experimental validation to iteratively optimize the antibody sequence. After the first round of optimization, the antibody's affinity for Antigen A improved fourfold. Incorporating all experimental data into the algorithm model for fine-tuning, the second round of optimization yielded an antibody with a sevenfold increase in affinity for Antigen A while maintaining low affinity for Antigen B.
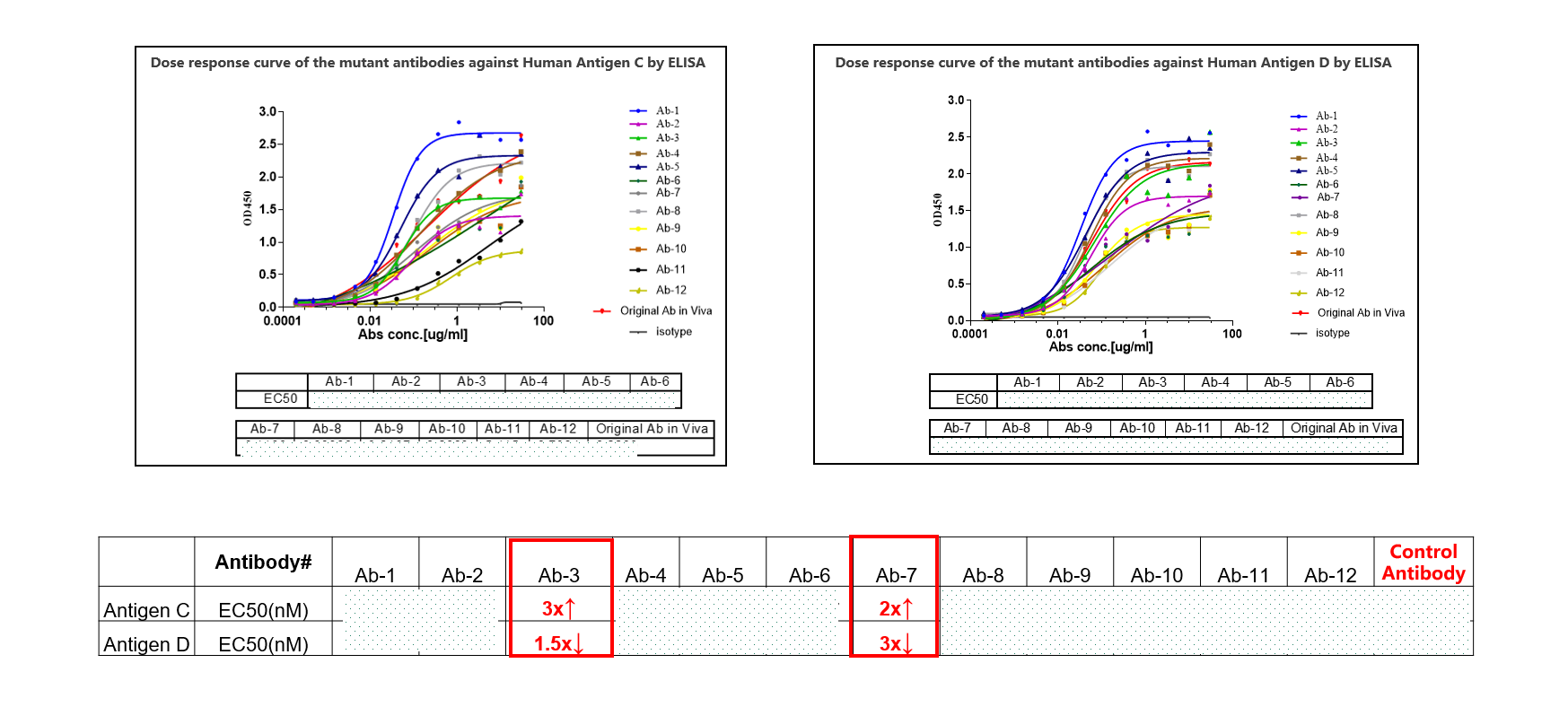
The main objective in this case is to increase the antibody's affinity for Antigen C and reduce its affinity for the highly homologous Antigen D. Identifying the subtle molecular differences presented a greater challenge due to the high similarity between Antigens C and D. Viva Biotech’s team began by analyzing the structures of the antibody-antigen C/D complexes to compare and contrast their binding modes. Using AI models, they predicted the effects of CDR site mutations on the affinities for Antigens C and D. Based on these predictions, the research team engineered a series of optimized antibody molecules and performed wet-lab experiments to screen and validate their affinities. Ultimately, through iterative feedback between the AIDD model and experimental data, they achieved an optimized antibody with a twofold increase in affinity for Antigen C and a threefold decrease in affinity for Antigen D.
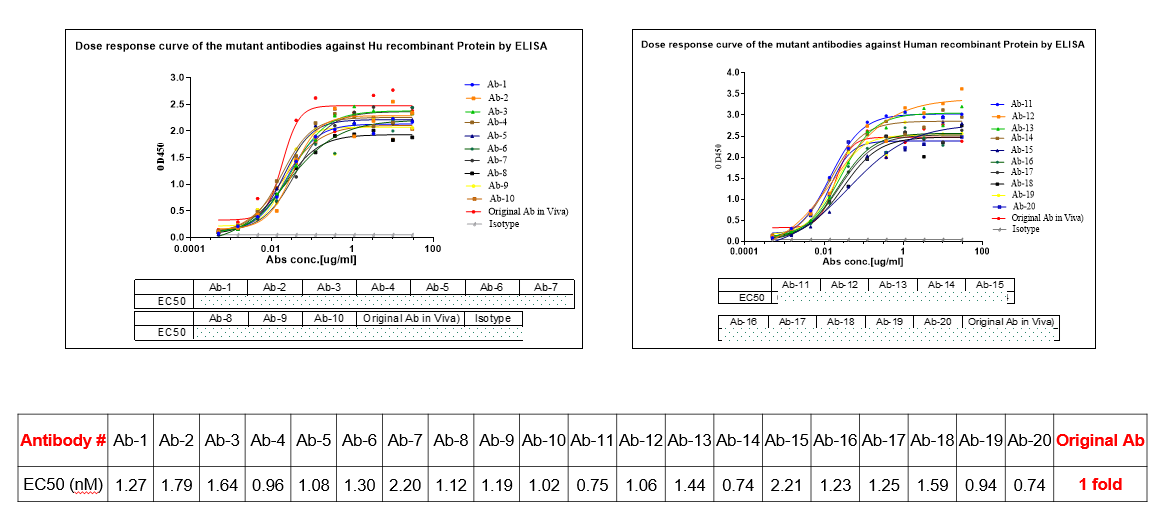
In the third case, clients require the redevelopment of expired patent antibodies for new applications or the design of bispecific antibodies. Viva Biotech addresses this by optimizing and modifying the CDR regions of the original antibody sequences. Since the CDR regions determine the antibody's specificity, altering these sequences can circumvent original patent restrictions and create new, patentable antibody molecules. The research team employed a cyclic optimization strategy combining AIDD/CADD with experimental validation. They first used AI models to predict the effects of various CDR amino acid mutations on antibody affinity and other physicochemical properties. Based on these predictions, they engineered a series of modified antibodies for experimental validation. In this case, Viva Biotech successfully obtained multiple optimized molecules with EC50 values comparable to or exceeding those of the original antibody. This demonstrated that the new antibody molecules retained high affinity while avoiding original patent restrictions.
In addition to its capabilities in monoclonal antibody development, Viva Biotech has expanded its expertise in bispecific antibody development. Viva Biotech has obtained authorization for Lonza's advanced bYlok® bispecific pairing technology, significantly enhancing their design and development capabilities. Lonza's bYlok® technology platform effectively addresses the mispairing problem of heavy/light chains, improving the production efficiency and quality of bispecific antibodies. It offers advantages such as high productivity, stability, and simplified processes.
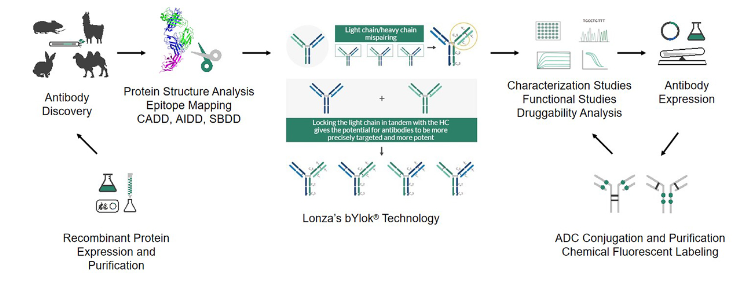
A license agreement with Lonza to obtain access to Lonza's bYlok® bispecific pairing technology.
With the successful implementation of these case studies, Viva Biotech has demonstrated its robust capabilities in antibody development services. This achievement is primarily attributed to the synergy of two core technology platforms: the powerful protein structure analysis platform and the advanced AIDD/CADD platform. The protein structure analysis platform integrates three complementary technologies—X-ray crystallography, cryo-EM, and HDX-MS—to meet the structural analysis needs at various stages. In 2023 alone, Viva Biotech successfully resolved over 16,000 protein/complex structures. By the end of 2023, they had delivered over 65,035 protein/complex structures involving more than 1,900 unique drug targets to their clients. These figures highlight the platform's efficiency and broad applicability. The AIDD/CADD platform, equipped with a high-performance computing center, supports extensive molecular dynamics simulations, free energy calculations, molecular docking, and efficiently applies various AI algorithms. This combination of structural biology, AIDD/CADD, and experimental validation significantly expedites antibody drug discovery and enhances success rates, paving a more efficient and precise path for the antibody drug R&D process.

