- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


Home / News / Events / Events detail

The 4th Global Conference & Exhibition on Biopharma Frontier Technology," co-organized by Tonacea and the National Center of Technology Innovation for Biopharmaceuticals (NCTI), and co-directed by four national associations/societies, took place from July 11-13, 2023, at Suzhou International Expo Center in Jiangsu, China. Dr. Jianhua Cai, Senior Vice President of Viva Biotech, was invited to attend the conference and gave a speech on the topic of “Sharing of new ligand PROTAC development technology”
"Screening of new E3 ligase ligands is necessary, especially the development of highly specific E3 ligase ligands, which is of great clinical importance."
--Dr. Jianhua Cai
Unlike small molecule inhibitors, PROTAC drugs work through a catalytic mechanism and do not need to bind to the disease-causing target for an extended period. This characteristic is expected to address the challenges of "undruggability" and "drug resistance" associated with traditional drugs. Dr. Cai discussed the cutting-edge research and development of PROTAC, highlighting that only a few E3 ligases, such as VHL, CRBN, IAPs, and MDM2, have been utilized in PROTAC design. However, there is a concern that drug resistance may emerge in PROTAC drugs developed based on existing E3 ligases. Therefore, the screening of new E3 ligases, particularly those with high potency, is necessary. Additionally, the screening of new E3 ligands is essential, emphasizing the importance of developing highly specific E3 ligase ligands for clinical applications.
Viva possesses advanced affinity screening technology platforms, including ASMS, Crystal soaking, SPR, and TSA screening technologies. These platforms can facilitate the development of new generation E3 ligase ligands or the screening of novel target protein binding molecules. ASMS technology stands out due to its high throughput, label-free nature, flexible screening capabilities, and rapid method development. It can be performed in solution, making it suitable for screening fragment libraries and large drug-like libraries. Dr. Cai provided an example of ASMS and SPR screening of molecular glues, which were specifically designed by Viva, illustrating the utility of these technologies.
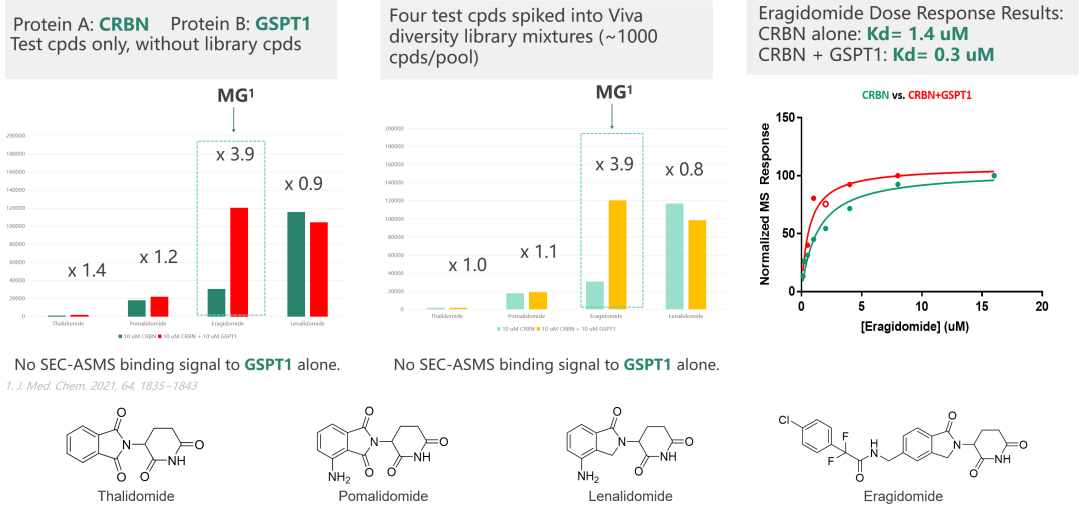
(Case study: SEC-ASMS Screening of MG)
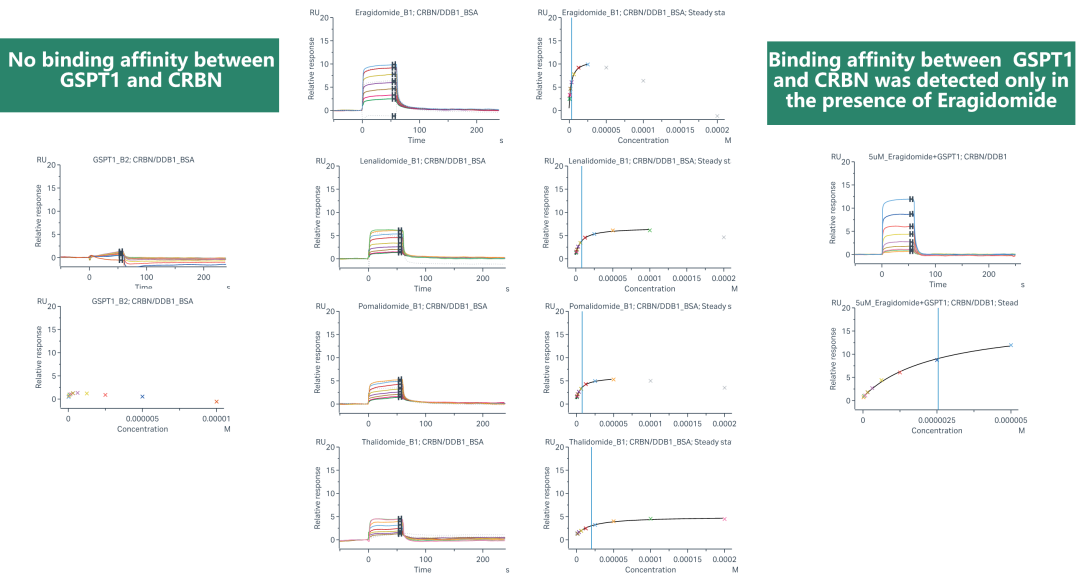
(Case Study: Binding Kinetics Study of MG with SPR)
In addition to the ligand screening technology, he also shared Viva's PROTAC platform, which covers a full range of biology and chemistry. By virtue of its strong protein structure research capability, Viva has accumulated more than 50 E3 ligases and delivered over 100 crystal structures of target protein-PROTAC-E3 ligase ternary complexes. In recent years, it has also established a method for the rapid analysis of CRBN ternary complex structures using Cryo-EM, with an average resolution of 3Å. Furthermore, Viva combines the Bioassay platform, chemical services, and DMPK to develop a mature drug discovery and development system. This system is complemented by CADD and AIDD to assist in the PROTAC molecular design and optimization process.
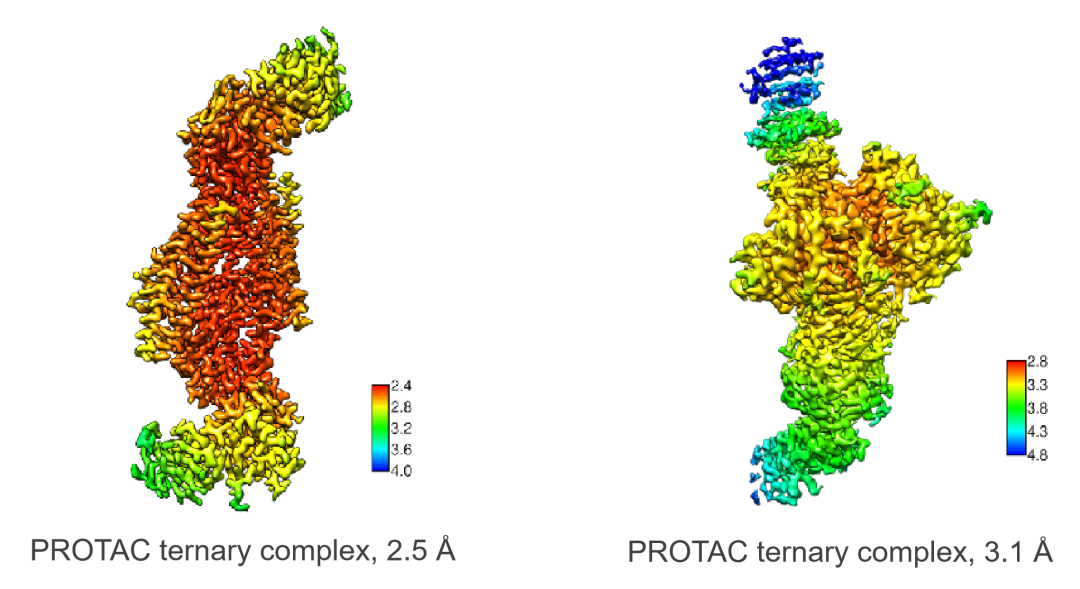
(Case study: PROTAC Structure Research with Cryo-EM)
At this conference, Viva also joined the “Tonacea Non-clinical Evaluation Alliance” and participated in the award ceremony as a member of the governing unit. The aim of the alliance is to bring together organizations with a vision and a sense of mission in the field of non-clinical evaluation in China. The goal is to explore new paths for drug development in China, promote the scientific formulation of non-clinical regulations and guidelines, cultivate more research, and development talents in the field of non-clinical evaluation, and improve the technical and management levels of new drug research and development in China. With the aim of embracing new opportunities and challenges brought about by changes in medicine, and collectively promoting the healthy development of new drug development, the conference will contribute to the advancement of new drug development in China.

During the exhibition, Viva's staff patiently received the visiting guests at the booth, shared with them Viva’s successful experience and professional insights in the field of new drug R&D and production, and fully demonstrated the one-stop comprehensive service capability, from early structure-based drug R&D to commercialized drug delivery.

Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us