Home / News / Events / Events detail
Over the past few years, a wide range of innovative small molecule drug discovery technologies have emerged, with Fragment-Based Drug Discovery (FBDD) becoming the mainstream approach. This development has injected new energy into biopharmaceutical innovation. On June 28th, we successfully hosted the "FBDD - Technology for FIC New Drug Discovery Breakthrough" event, bringing together industry pioneers and academic experts both online and offline. The event aimed to explore the cutting-edge technology and perspectives on FBDD comprehensively, to advance biopharmaceutical R&D to a new level. Here are the key insights from the panelists below for our readers.
(To watch the full video playback, please visit BiliBili.)
Dr. Han Dai, CIO and Head of Viva BioInnovator
FBDD – The Key to Driving New Opportunities for Innovative Drugs in an Era of Transformation

In recent years, small molecule drugs have faced competition from antibody/protein drugs and CGT in the pharmaceutical market. However, innovative technologies have rejuvenated small-molecule drug development. Dr. Han Dai has pioneered a novel small molecule screening technology platform known as FBDD. He emphasized that compared to traditional high-throughput screening (HTS), fragment compounds have smaller molecular weight, greater potential for optimization, and better fitting with protein binding sites, making them more likely to yield highly efficient lead compounds.
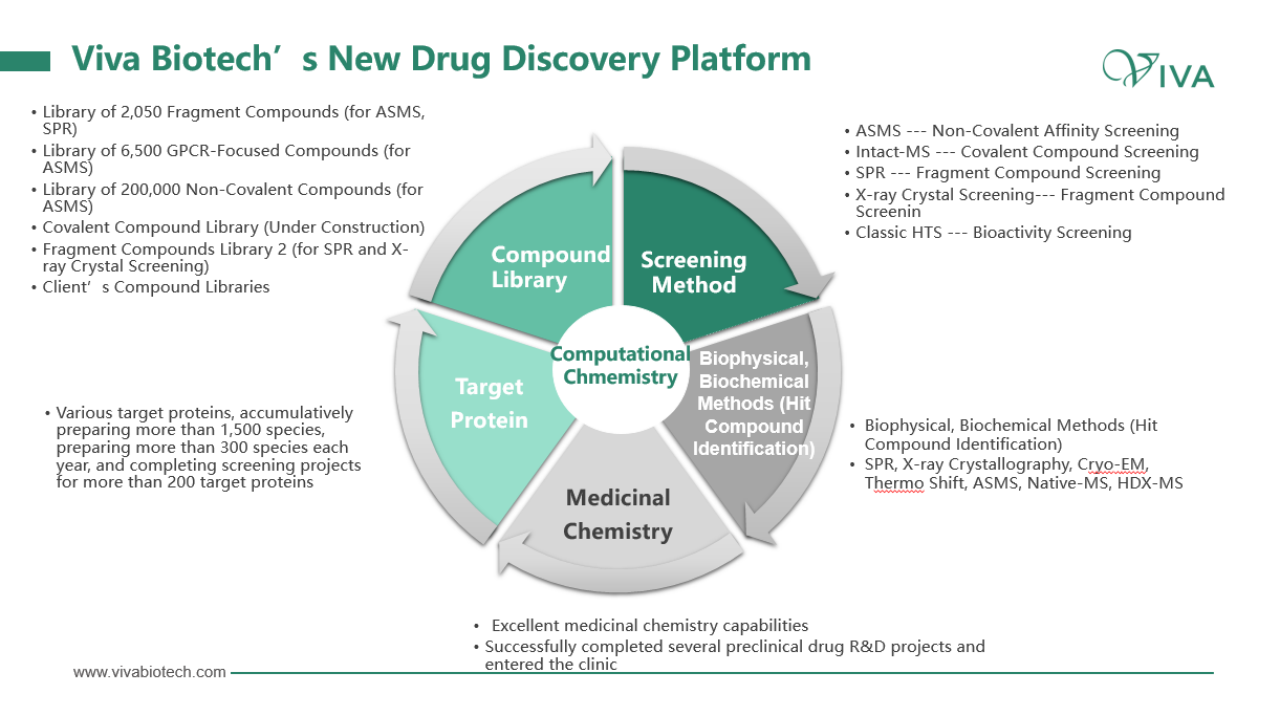
Dr. Dai further discussed two common optimization approaches in FBDD: fragment extension and fragment ligation method, using examples of targets such as MAT2A and Bcl-2. Of course, he also introduced Viva’s affinity screening platform, explaining the functionality of the platform in both non-covalent and covalent compound screening. Additionally, he highlights the utilization of various techniques such as ASMS, crystal immersion, SPR, Intact-MS, and TSA in specific scenarios.
By the end of 2022, Viva has delivered over 48,925 protein structures to customers, with approximately 14,534 newly delivered structures in the same year. Furthermore, Viva had conducted research on more than 1,878 targets, and 68 new targets were delivered in 2022. Finally, Dr. Dai illustrates the application of Viva’s new drug discovery technology platform through the case study of a small-molecule allosteric inhibitor of PCSK9 and the discovery of a novel mechanism for anti-cancer drug development.
Dr. Qisheng Wang, Researcher at Shanghai Advanced Research Institute, Chinese Academy of Sciences
The Application of Synchrotron Light Source in FBDD Development Strategy

As we are aware, the emergence of Synchrotron Light Sources (SSRF) has significantly advanced crystal X-ray diffraction. In his presentation, Dr. Qisheng Wang discussed the layout of the Shanghai Synchrotron Radiation Facility (SSRF) beamlines, which currently encompasses approximately 40 beamlines in operation. These include the first crystallography beamline, BL17U1, the BL17U1 relocation beamline (BL02U1), the biosafety level-2 protein crystallography beamline (BL10U2), the high-performance membrane crystallography beamline station (BL17UM), and the microbeam Laue diffraction endstation (BL03HB).
Dr. Wang also highlighted the high-throughput experimental technology employed at the crystallography beamline. SSRF utilizes new detectors, sample robotics, and integrated software to ensure the efficiency and quality of crystal diffraction data. A comparison between 2016 and 2017 revealed notable improvements, with acquisition time decreasing from 30 minutes to 30 seconds ideally, and the number of data sets increasing from 4,867 to 10,096. Furthermore, he shared insights on the ongoing construction of the FBDD experimental platform at SSRF.
Dr. Bing Xiong, Researcher and Doctoral Supervisor of Shanghai Institute of Materia Medica. Chinese Academy of Sciences
Development of a Novel Drug Targeting Protein Post-Translational Modification System Using the Fragment-Based Drug Discovery Strategy

How to develop drugs to treat diseases has long been intertwined with the complexities of the human body? The intricacies of human complexity lie not only in the sheer number of genes but also in how these genes are utilized, the myriad chemical changes in proteins, and the regulation of protein production through non-coding regions of the genome.
From a drug development perspective, Dr. Bing Xiong shed light on his choice of protein post-translational modification systems as targets and explores the advantages of the FBDD approach. In comparison to high-throughput screening methods, FBDD stands out with its high diversity and small library capacity of fragment molecular libraries, resulting in higher ligand efficiency despite the lower activity. The applications of FBDD can be divided into two categories: conventional fragment libraries for drug discovery and covalent fragment libraries for target discovery. FBDD also offers opportunities to examine target targetability, discover multiple binding sites, and design protein degraders in combination with the PROTAC strategy.
Furthermore, Dr. Xiong mentioned that 7% of new clinical molecules during 2018-2021 were obtained from fragment screening, and this percentage is increasing. He also highlighted FBDD as a new mode of subtractive thinking in drug discovery.
During the speech, Dr. Xiong also introduced the FBDD technology platform established by his group, equipped with a fragment library of around 2,000 molecules, comprehensive screening methods, and experience in developing multiple targets. Several case studies illustrate the success of FBDD in post-translational modification target research. For instance, the study of the inhibitor of Ras lipidation-transport protein PDEδ led to the development of a new structure type through FBDD, with optimal compound reaching an impressive 26 nM and exhibiting 2,000 times higher activity.
Finally, Dr. Xiong emphasized that the FBDD development strategy relies on the support of crystalline structure of complex and subsequent chemical structure modifications. He provided examples of the rapid application of Fragment link, Fragment growth, and Fragment merge to enhance compound activity. FBDD proves to be an efficient and economical method for discovering new binding sites (orthosteric and allosteric sites) and synergizing with PROTAC/RiboTAC technology in drug development.
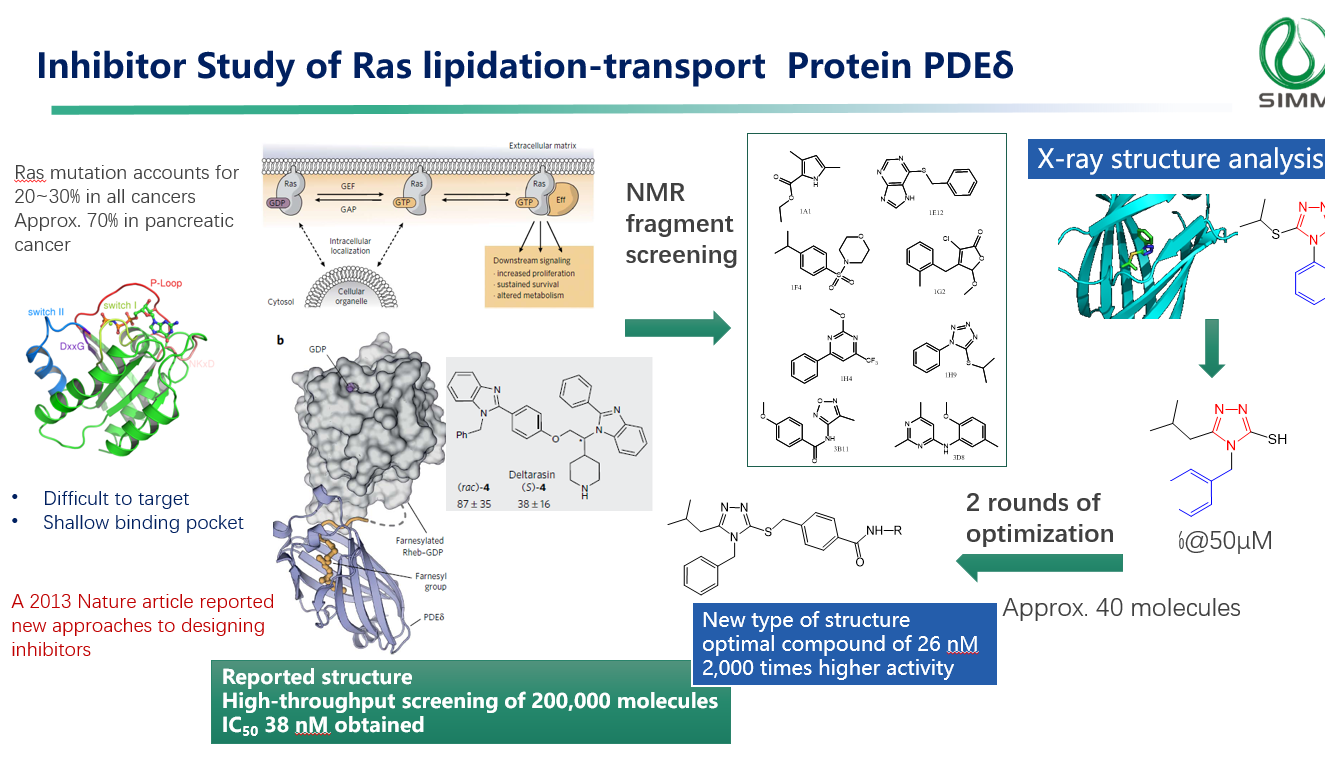
(Case: Inhibitor study of Ras lipidation-transport protein PDEδ)
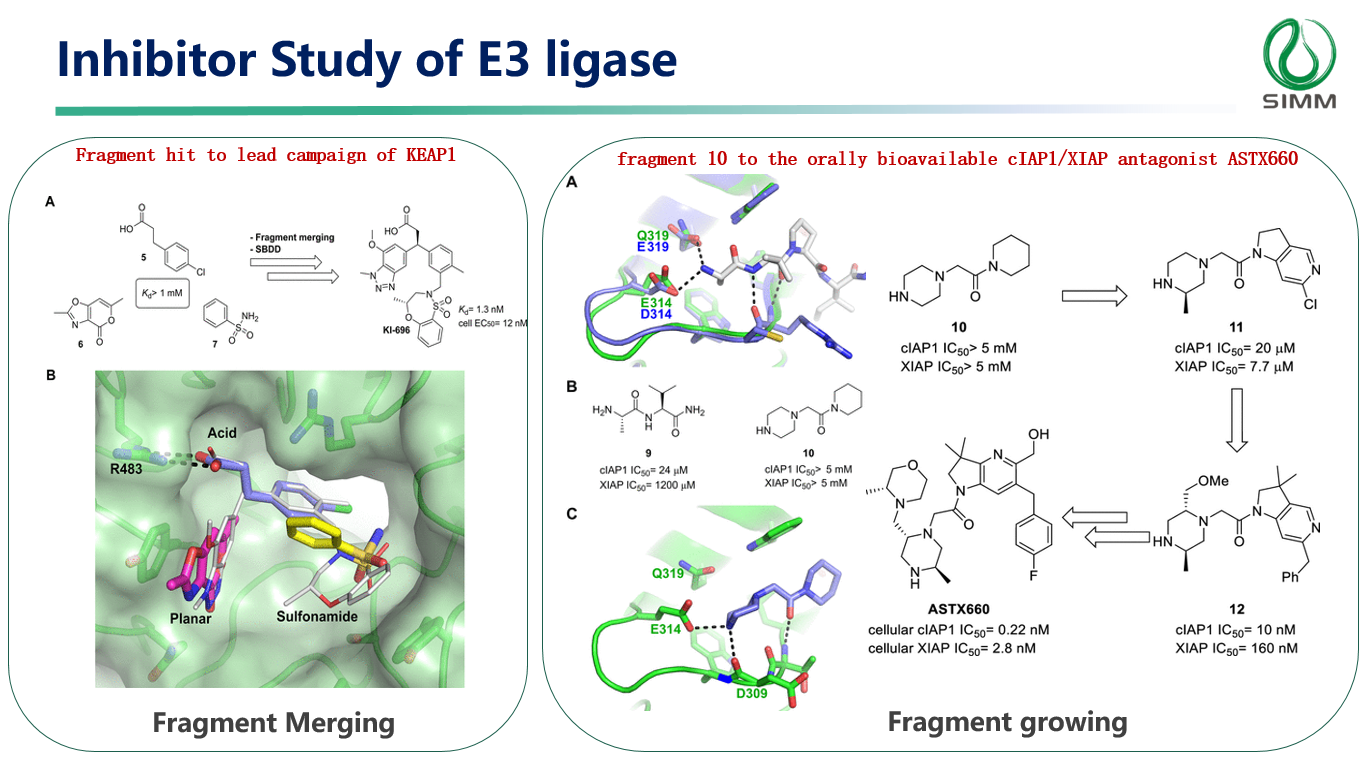
(Case: Inhibitor study of E3 ligase )
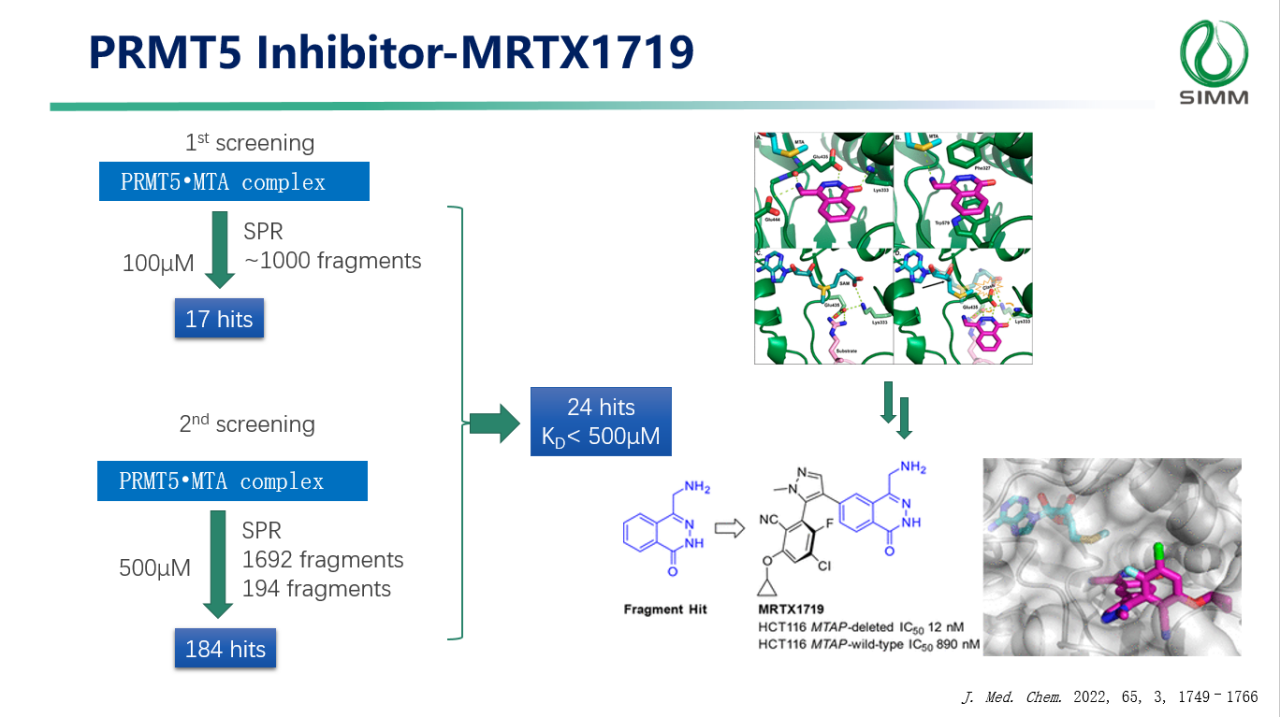
(Case: PRMT5 Inhibitor-MRTX1719)
Panel: Exploring Drug Discovery - Drug Screening Methods and Case Studies

During the panel discussion, Dr. Han Dai, CIO of Viva Biotech and Head of Viva BioInnovator, served as the host. The esteemed panel consisted of five industry experts: Dr. Yechun Xu, Researcher and Doctoral Supervisor at Shanghai Institute of Materia Medica. Chinese Academy of Sciences; Dr. Zhixiong Ye, CSO of Viva Biotech; Dr. Zengquan Wang, Founder, Chairman, and General Manager of TechnoDerma Medicines; Dr. Yikai Wang, Founder and CEO of Keen Therapeutics; Dr. Bing Xiong, Researcher and Doctoral supervisor at Shanghai Institute of Materia Medica. Chinese Academy of Sciences (from left to right).
The focus of the discussion was " Exploring Drug Discovery - Drug Screening Methods and Case Studies " The panelists engaged in an insightful and productive exchange of ideas and perspectives on the following four topics.
Q1:In recent years, FBDD has played a significant role in the development of six FDA-approved drugs and over 50 clinical compounds. Let's briefly discuss the types of targets to which FBDD technology can be applied and the commonly used screening methods and compound libraries.
Dr. Zengquan Wang: From an industry perspective, the efficient identification of hits and their progression to clinical development is of utmost importance. Small companies often face limitations in screening large compound libraries, which is where FBDD demonstrates clear advantages. The fragment library in FBDD can consist of hundreds, thousands, or even tens of thousands of compounds, providing flexibility without restrictions.
However, applying FBDD to challenging targets like membrane presents technical difficulties. Firstly, the preparation of membrane protein targets itself is a complex task. Subsequently, integrating them into the Fragment-based screening process, identifying hits, and verifying their efficacy requires considerable time and effort. Therefore, companies must carefully and comprehensively consider multiple factors, including target selection and library selection, when choosing a specific technology.
Dr. Bing Xiong: Evidence suggests that incorporating a greater number of SP3- hybridized carbons results in a more diverse compound library with enhanced stereochemistry, thereby increasing the likelihood of identifying active fragments for complex targets. In the case of GPCR targets, he believed that the advancements in Cryo-EM technology will facilitate the broader application of FBDD in this domain.
Q2: What differentiates FBDD from other screening methods and what are its strengths and limitations? Additionally, what drug development techniques can aid in the subsequent optimization process for FBDD?
Dr. Yechun Xu: Each screening method has its advantages, disadvantages, and specific areas of applicability, requiring a careful analysis of the specific situation at hand. In the context of FBDD, it encompasses various screening methods and is not limited to a single approach. However, it is important to note that the affinity of fragment hits identified through FBDD may be weaker compared to methods like HTS or DEL. Therefore, the focus should be on screening to identify weak binding fragments, particularly for challenging targets such as GPCRs, where traditional enzyme activation methods may not be applicable. To address this, it is advisable to develop multiple assays for cross-validation, such as SPR, mass spectrometry, TSA, and others, to gain a comprehensive understanding of fragment binding. Furthermore, if possible, utilizing crystal-based FBDD screening is a favorable strategy as it provides clearer insights into the fragment binding pattern, allowing for more effective subsequent structure optimization.
Dr. Zengquan Wang: FBDD faces a significant challenge in terms of its high false positive rate. While FBDD has shown success in studying relatively easier targets, it becomes more demanding for difficult drug targets. Notably, all the FDA-approved drugs developed using FBDD so far are kinases, which offer favorable binding sites, making it easier to identify hits. One unique aspect of FBDD is its ability to identify both active center inhibitors and allosteric inhibitors, a feat that is challenging with traditional screening methods.
When selecting a technology for drug development, careful consideration should be given to the specific target, screening method, and subsequent processes involved. Additionally, the importance of crystal structure in FBDD cannot be overstated.
Q3: FBDD exhibits great potential in advancing drug development for difficult drug targets. What is your assessment of the current level of FBDD's application in addressing difficult drug targets? Can it serve as an optimal screening method for both difficult drug targets and non-protein targets like PROTAC and RNA garnering significant attention in recent years?
Dr. Bing Xiong: He believes that FBDD is a natural and effective combination with PROTAC technology for challenging targets. The small molecular weight of the fragment remains manageable even when linked to a linker or E3 ligase, resulting in relatively fewer PK optimization challenges. Conversely, complex molecules with a linker and E3 ligase can lead to excessive molecular weight. Previous studies have shown that while preclinical small animal PK phases were favorable, significant PK parameter changes occurred in larger animals, making PK prediction in humans challenging and risky. By employing the FBDD strategy, the molecular weight can be well controlled, contributing to consistent PK profiles.
Q4: As AI technology continues to advance, the drug development industry has witnessed a surge in attention and investment. When considering their specific application, how do you envision the synergistic potential of AIDD/CADD and FBDD? Furthermore, what are the future development trends in this field?
Dr. Yikai Wang: He believes that both AIDD and CADD rely on solving equations and utilizing computational power. When it comes to their synergistic application with FBDD, he highlighted the development of Keen Therapeutics’s fragment-based computing platform since 2020. The unique advantage of computer-based approaches lies in their capacity to efficiently explore vast chemical spaces and identify molecules with high potential for protein binding. He also disclosed that Keen currently possessed the capability to screen and validate fragment-based wet lab experiments. By leveraging hydrogen bonds within fragments and protein eutectics as a starting point for calculations, the accuracy of molecular design and the efficiency of optimization iterations can be significantly enhanced. This combination holds the potential to make FBDD a more effective tool for drug discovery.

