- About Us
-
CRO Services
- PROTAC/Molecular Glue Services
- Protein Preparation and Ternary Complex Structure Determination
- PROTAC/Molecular Glues Screening
- PROTAC Ternary Complex Kinetics (SPR)
- PROTAC Degradation Assays and Ternary Complex Assays
- PROTAC Molecule Design and Synthesis
- ADME & PK/PD Studies of PROTAC Molecules
- AIDD/CADD PROTAC Design
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us


Phospholipase C (PLC) plays a pivotal role in cellular signal transduction. Phospholipase C gamma 2 (PLCγ2, encoded by gene PLCG2) is a membrane- associated enzyme that catalyzes the conversion of 1-phosphatidyl-1D-myo-inositol 4,5-bisphosphate (PIP2) to 1D- myo- inositol 1,4,5- trisphosphate (IP3) and diacylglycerol (DAG) using calcium as a cofactor. The PLC family is known to include at least four isoforms: PLCβ、PLCγ、PLCδ and PLCε, with their activity primarily regulated through phosphorylation. Among these, PLCγ2 stands out for its critical role in cellular signal transduction downstream of various membrane receptors. This protein plays a critical role in cell signaling downstream of various membrane receptors, with its multidomain inhibitory region being essential for regulating its activity. For a long time, scientists have been intrigued by the precise mechanisms through which these domains modulate PLCγ2 activity.
(Figure1:Image come from Science official website)
On November 29, 2024, the journal Science Advances published a research paper titled "The crystal and cryo-EM structures of PLCγ2 reveal dynamic interdomain recognitions in autoinhibition" offering exciting progress in addressing this scientific mystery. This study was co-conducted by a research team led by Dr. Dongming Qian, Vice President of Protein and Structural Biology at Viva Biotech. Leveraging the company's state-of-the-art protein structure elucidation platform, the team provided critical support for uncovering the molecular regulatory mechanisms of PLCγ2.
The study utilized structural biology techniques to successfully determine three structures of human PLCγ2 in its autoinhibited state. These structures suggested dynamic interactions at the autoinhibition interface, involving the conformational flexibility of the Src homology 3 (SH3) domain in the inhibitory region and its previously unknown interaction with the carboxy-terminal helical domain in the core region. Importantly, the study also determined the binding structure of PLCγ2 with the kinase domain of fibroblast growth factor receptor 1 (FGFR1), elucidating the recognition mechanism of the nSH2 domain in PLCγ2's inhibitory region for FGFR1. These findings provide unprecedented structural insights into the regulation of PLCγ2 and will significantly advance future studies of its complete activation process.
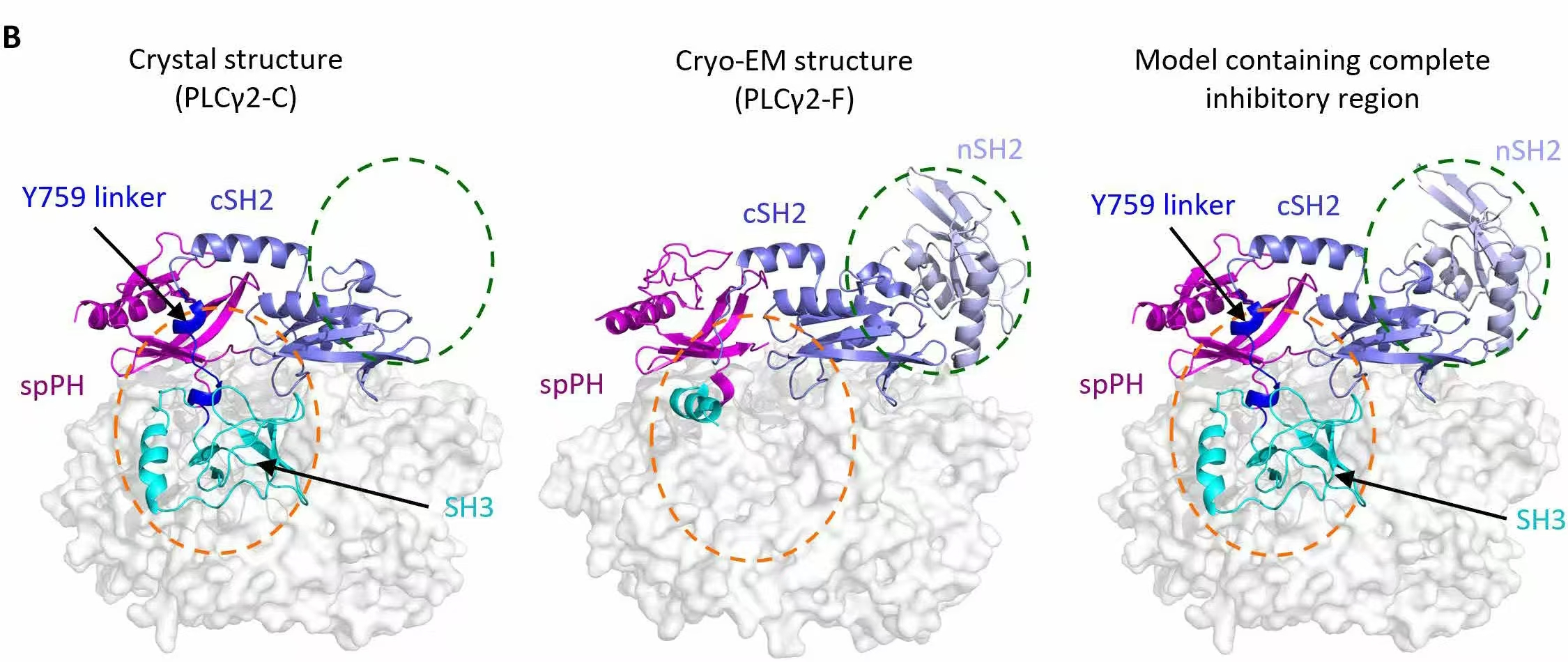
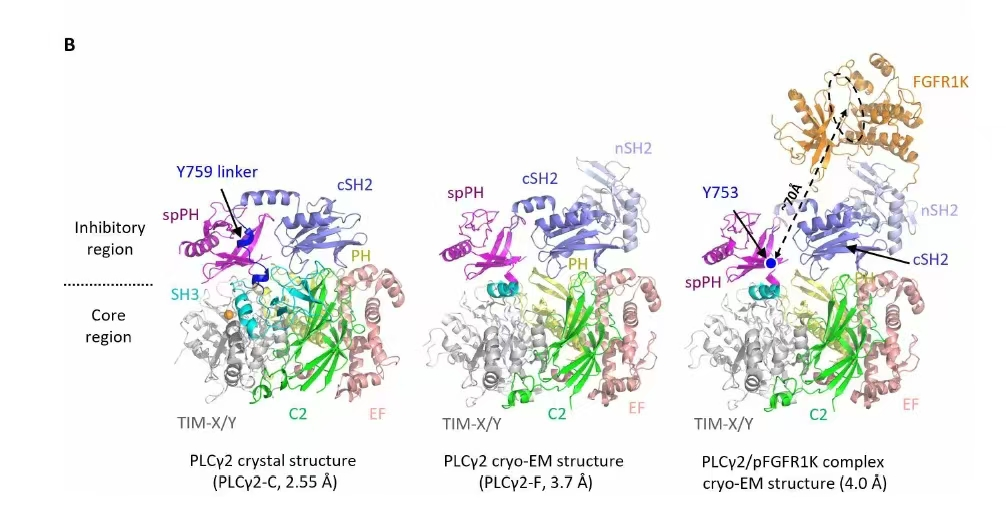
(Figure 2: Image comes from the research, the X-ray crystal structure presented in this study originated from research conducted independently by Viva Biotech)
In this research, Viva Biotech's advanced protein expression platform (spanning E. coli, insect, and mammalian cell systems) and exceptional structural discovery services (including crystallization and X-ray structure determination) provided strongly technical support. The Viva Biotech scientific team successfully resolved the 2.55 Å crystal structure of PLCγ2-C, revealing key molecular interactions between the core inhibitory region and the catalytic core (Figure 2). Furthermore, leveraging full-length PLCγ2 and FGFR1 proteins produced by Viva Biotech, in collaboration with research institutions, the team resolved the 3.7 Å structure of PLCγ2-F and the 4.0 Å PLCγ2/pFGFR1 complex structure (Figure 2). These structural discoveries offer crucial insights into the regulatory mechanisms of PLCγ2 and its pivotal role in cellular signaling pathways.
This study not only makes a significant impact in scientific understanding but also stands as a testament to Viva Biotech's exceptional expertise in the field of structural biology. Since its founding in 2008, the company has been dedicated to advancing this cutting-edge field, building high technological barriers to help global clients overcome world-class challenges in structural biology. As of June 30, 2024, Viva Biotech has delivered over 74,000 protein structures to clients worldwide, with cumulative research covering more than 2,000 drug targets—securing its position as a global leader in protein structure elucidation. In the future, Viva Biotech will continue to build on its technical strengths, fostering consistent progress in biopharmaceutical innovation and contributing to impactful research advancements.
For further details on the paper, please refer to:
Young-Cheul Shin et al. ,The crystal and cryo-EM structures of PLCγ2 reveal dynamic interdomain recognitions in autoinhibition.Sci. Adv.10,eadn6037(2024).DOI:10.1126/sciadv.adn6037
If you are interested in Viva Biotech's Protein Research Platform, please reach out to our expert team via email at info@vivabiotech.com for more detailed communication.
Copyright © Viva Biotech All Rights Reserved. 沪ICP备19036061号
- About Us
-
CRO Services
BackCRO ServicesService & Technology
- CDMO Services
- EFS Business
- News
- Careers
- Investor Relations
- Contact Us