On November 10, the inauguration ceremony of Viva Biotech's headquarters took place in Zhoupu, Pudong New Area, Shanghai. The people in attendance included: Mr. Qiang Wu, Deputy District Mayor of Shanghai Pudong New Area; Mr. Gang Ling, Chief Engineer of Shanghai Pudong Technical and Economy Committee ; Mr. Kairong Wang, Deputy General Manager of Shanghai Zhangjiang (Group) Co., Ltd.; Mr. Jianping Wu, General Manager of Shanghai Free Trade Zone Fund; Ms. Jiayao Xuan, Deputy General Manager of Shanghai Zhangjiang Technology Venture Capital Co., Ltd.; Ms. Tian Shen, Mayor of Youchegang Town, Xiuzhou District, Jiaxing City; Mr. Weidong Yu, Chairman of Jiaxing Linhu Holding Group Co., Ltd. More than 100 guests, including entrepreneurs, scientists, and investment institutions attended the event to witness the important milestone. Click here to watch the video.
Mr. Qiang Wu, Deputy District Mayor of Shanghai Pudong New Area, began the ceremony with a speech. He stated: "I am very pleased to participate in the completion ceremony of Viva’s headquarters and to witness their achievements along the way. Today, Viva headquarters, also nicknamed the "aircraft carrier," officially ushered in its voyage. After injecting new impetus into Viva, it will start a new and vigorous journey. The biomedical industry is one of the industries Pudong has been vigorously developing for a long time. Pudong New Area will continue to build an international, first-class business environment that supports a global biomedical industry hub, attracting more high-tech biomedical companies to settle here and accelerating the construction of an innovative epicenter for the industry."

(Mr. Qiang Wu, Deputy District Mayor of Shanghai Pudong New Area)
Dr. Cheney Mao, Chairman and CEO of Viva Biotech, expressed his gratitude to all the leaders and guests. He remarked, "Viva Biotech and the Pudong New Area Government have the same development philosophy and goals for the biomedical industry. The Zhoupu area has a good business environment and a concentration of talent, with huge development space and potential. With the completion of Viva headquarters in Zhoupu, we have ushered in another important milestone in the company’s development. The headquarters integrate the innovative drug R&D center and corporate offices with a total area of 40,000 square meters. It has a first-class R&D team, a high-end R&D laboratory, and an advanced technology platform. The one-stop CRO/CDMO service chain will collectively promote the high-quality development of the biomedical industry."

(Dr. Cheney Mao, Chairman and CEO of Viva Biotech)
During the ceremony, Mr. Qiang Wu, Mr. Gang Ling, Mr. Kairong Wang, Mr. Jianping Wu, Dr. Cheney Mao, and Dr. Derek Ren, came to the stage to cut the ribbon for the completion of the headquarters.
After the inauguration ceremony, Dr. Derek Ren, Executive Director and President of Viva Biotech, and Dr. Jianguo Ma, Senior Vice President of Viva Biotech and CEO of Langhua Pharmaceuticals, introduced Viva's new, one-stop drug R&D and production services in-depth at the technology and services event.
Dr. Ren first reviewed Viva's past and current developments, then elaborated on the broad applications of Viva's high-end technology around the three major themes of "MOA research, innovative drug screening strategies, and emerging technologies for new drug research and development." Viva uses an efficient protein expression system, powerful protein structure research, and HDX technology to explore the mystery of molecular mechanisms. Using unique affinity mass spectrometry technology, ASMS and SPR, combined with the bioassay platform, chemical services, and DMPK, Viva can develop a mature drug research and development system, ultimately helping the R&D process to advance quicker and more efficiently. In addition, Dr. Ren said that in the wave of new global drug R&D, Viva has always maintained the same rate of innovation and has implemented multiple emerging technology platforms such as PROTAC technology, computer-aided drug design (CADD), and therapeutic antibody discovery. Efficient collaboration with technology platforms will continue to improve capabilities and efficiency.

(Dr. Derek Ren, Executive Director and President of Viva Biotech)
Dr. Jianguo Ma explained the development of the Viva subsidiary, Langhua, starting from raw materials to pharmaceuticals. He examined the three major R&D centers' significant achievements: a first-class core leadership team, a unique API and preparation technology platform, sufficient production capacity, and a reliable GMP and EHS management system. He cited several successful cases to share the advantages of a one-stop drug CDMO service in process development, scale-up, production, and IND declaration at various clinical stages.

(Dr. Jianguo Ma, Senior Vice President of Viva Biotech and CEO of Langhua Pharmaceuticals)
After the session, half of the guests visited the key laboratories and equipment in the headquarters building with Viva's tour guides. The newly completed headquarters has an industry-leading protein preparation and purification platform, a crystal structure research center, a high-end cryo-electron microscopy platform, drug screening platform (ASMS, HDX, SPR), an advanced bioassay platform, a medicinal chemistry area, and therapeutics area. It also includes a series of complete early drug R&D operation facilities, such as an antibody discovery platform and a CMC/CDMO research and development center. The center covers small molecule research, development, and production, including four GMP synthesis areas, two GMP D-level clean areas, and one formulation GMP area.
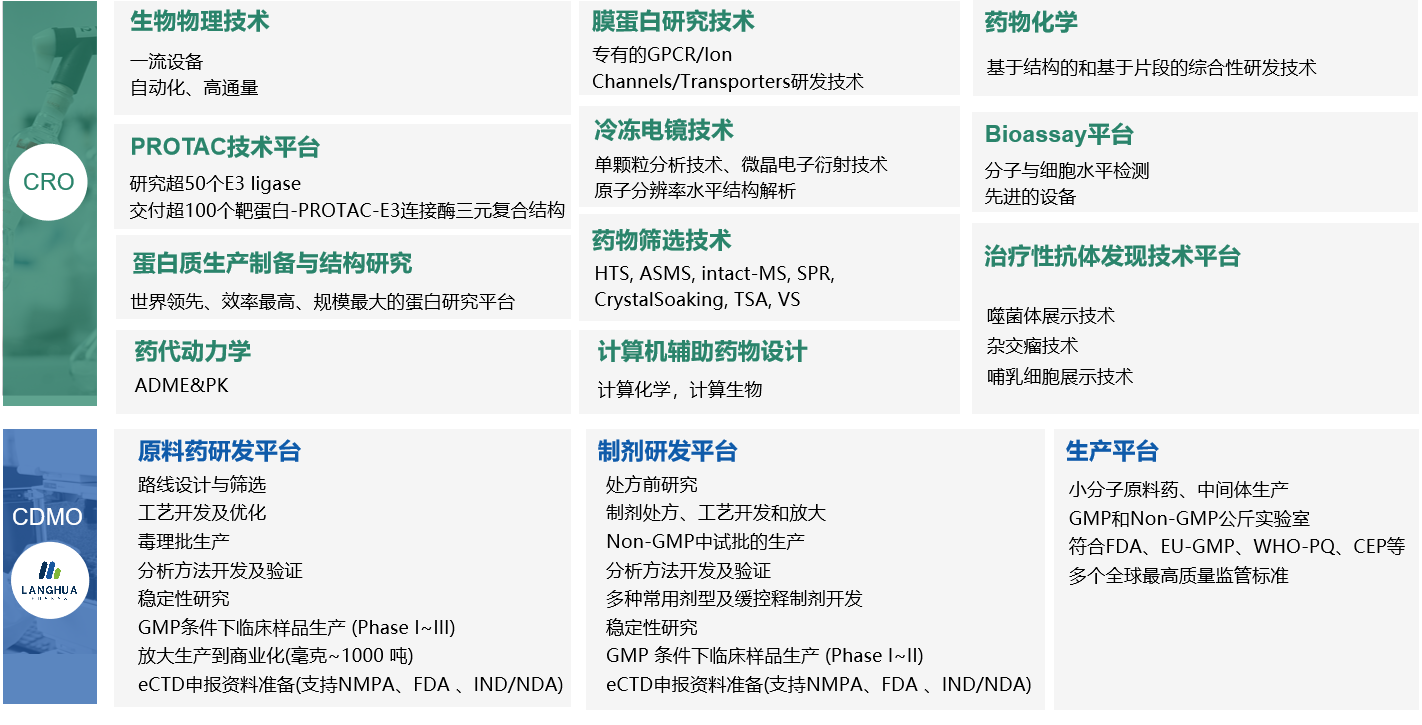
(Viva's One-Stop Service Platform from Early Structure-Based Drug Development to Commercial Drug Delivery)

(Guests Visit the Laboratories)
At the same time, the other guests who stayed at the venue had thorough discussions and exchanges with experts in the six technical areas of protein preparation, protein structure analysis, drug screening, biological activity, chemistry, and CMC, where a knowledgeable exchange of ideas happened.

(Networking and Discussion Among the Guests)
The completion of Viva Biotech's headquarters is an integral part of its expansion and strategy, and it has enhanced the company's comprehensive R&D capabilities. Going forward, Viva will continue to rely on its unique advantages in structure-based drug development (SBDD) to build and continuously improve technical barriers, improve efficiency, strengthen the one-stop drug R&D and production service platform, and deepen the relationship between the CRO and CDMO business. Viva strives for synergy and actively builds an open cooperation platform and a win-win ecosystem for global biomedical innovators.

